April 2024: In This Issue
Drug Topics
APRIL 9, 2024
See what's trending in pharmacy with a preview of Drug Topics' April issue.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

 issue
issue 
Drug Topics
APRIL 9, 2024
See what's trending in pharmacy with a preview of Drug Topics' April issue.

Drug Topics
APRIL 30, 2024
Don’t miss out on the latest pharmacy insights in the Drug Topics April issue.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Fierce Healthcare
FEBRUARY 22, 2024
Change Healthcare is mitigating a "cybersecurity issue" that began Wednesday, and details remain scant. Change Healthcare is mitigating a "cybersecurity issue" that began Wednesday, and details remain scant.

European Pharmaceutical Review
APRIL 29, 2024
Articles included in Issue 2 of European Pharmaceutical Review – our cell and gene therapy focus – include: CELL & GENE THERAPY Proper planning prevents CMC disasters for cell and gene therapies Drew Hope, Ryan Guest and Clare Blue eXmoor Pharma SUPPLY CHAIN Fresh or frozen?

Drug Topics
APRIL 15, 2024
See what's trending in pharmacy with a preview of the Total Pharmacy April issue.

Drug Topics
APRIL 23, 2024
Abeona Therapeutics’ pz-cel is an investigational autologous, COL7A1 gene-corrected epidermal sheet therapy for the treatment of recessive dystrophic epidermolysis bullosa.

European Pharmaceutical Review
MARCH 13, 2024
FDA inspections Identification of data integrity deviations Of the 70 Warning Letters issued by the US Food and Drug Administration (FDA) so far in 2024, three have identified data integrity issues at pharmaceutical manufacturing sites outside the US. In a letter issued to China-based Sichuan Deebio Pharmaceutical Co.

Drug Topics
OCTOBER 10, 2022
A look at what’s to come in the October issue of Drug Topics®.

Fierce Pharma
APRIL 5, 2024
Amid ongoing drug shortages and other headline-grabbing issues that fall under the FDA’s purview, the House Committee on Oversight and Accountability is putting the agency’s commissioner Robert Cal | The April 11 hearing could be contentious, as the committee wants to “hold the commissioner accountable" for the FDA's action on drug shortages, (..)

pharmaphorum
MAY 1, 2024
Discover why capacity remains a critical issue in the industry. Explore the challenges in weight loss treatments despite the billions spent on clinical trials for weight loss drugs.

European Pharmaceutical Review
MARCH 1, 2024
This Issue was brought to you in partnership with Fujifilm Irvine Scientific: The post <em>European Pharmaceutical Review </em> Issue 1 2024 appeared first on European Pharmaceutical Review.
Pharmacy Times
JANUARY 3, 2023
In addition to the physical symptoms of long COVID, patients have reported vocal, verbal, and cognitive issues that disrupt their ability to communicate.
European Pharmaceutical Review
MARCH 11, 2024
The manufacturer Olympus Corporation has announced the rescission of an earlier acquisition deal due to reported data integrity issues of medical device products from Taewoong Medical Co., Data integrity issues The concerns around these products were found after the closing of the deal, Olympus noted. Up to $114.5

Drug Topics
MARCH 5, 2024
Health care professionals, industry leaders, and politicians gathered for a virtual roundtable to discuss the state of the PBM industry.

Fierce Healthcare
FEBRUARY 20, 2024
A year after hitting the pause button on a multi-billion-dollar health tech project at the Department of Veterans Affairs, federal lawmakers continue to have serious concerns about pharmacy softwar | A year after hitting the pause button on a multi-billion-dollar health tech project at the Department of Veterans Affairs, federal lawmakers continue (..)
Pharmaceutical Technology
MAY 2, 2024
Emerging research from the ESCMID Global 2024 conference suggests that antifungal resistance, a global issue, is on the rise.

Hospital Pharmacy Europe
APRIL 4, 2024
The importance of pharmacy professionals signposting children and young people experiencing gender identity issues such as gender incongruence or dysphoria to appropriate support services has been emphasised by the General Pharmaceutical Council (GPhC).

Fierce Pharma
APRIL 10, 2024
In a four-observation warning letter issued this week, the U.S. It usually goes without saying that your pharmaceutical production workers need to be gowned and gloved while handling drug materials inside clean rooms. |
STAT
APRIL 24, 2024
After months of deliberation, the Colombian government has issued a compulsory license for an HIV medicine, the first time the country has taken such a step, one that also marks a significant move in the increasingly global battle over access to medicines.

European Pharmaceutical Review
NOVEMBER 9, 2023
Issue 5 of European Pharmaceutical Review features articles on manufacturing, development and quality control of biopharmaceuticals from antibodies to mRNA and cell and gene therapies. We also explore opportunities for outsourcing of regulatory activities, the challenges of paediatric drug formulation and much more.

Pharma Marketing Network
JULY 26, 2023
However, in recent years, there has been a noticeable decline in the number of these letters being issued. Shifting Enforcement Priorities: One possible reason for the decrease in FDA-issued marketing violation letters is a shift in the agency’s enforcement priorities.

Pharmacy Times
OCTOBER 19, 2023
Latinx teens suffering with mental health issues are at risk of developing cardiovascular conditions in their future.
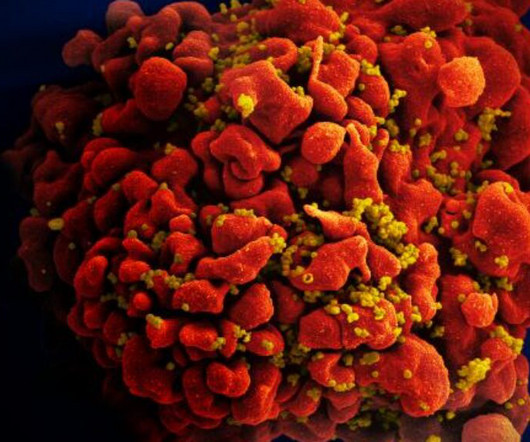
STAT
FEBRUARY 6, 2024
The Colombian government has taken a significant step toward issuing a compulsory license for a widely used HIV treatment, the latest instance in which cash-strapped governments battle with the pharmaceutical industry over the cost of medicines.

Drug Store News
AUGUST 10, 2023
Pharmacy legislation covering important issues, such as PBM reform and scope of practice, is gaining momentum at the state and federal levels. Here are the ones to watch.

European Pharmaceutical Review
FEBRUARY 21, 2023
Included in this issue of European Pharmaceutical Review : FOREWORD Accelerating drug development David Elder, David P Elder Consultancy RAMAN SPECTROSCOPY Shining a light on Raman for microbiological analysis Tim Sandle, Bio Products Laboratory LIMS Overcoming barriers to capturing data in a laboratory Samantha Kanza, University of Southampton ENVIRONMENTAL (..)

DiversifyRx
SEPTEMBER 23, 2022
When you have owned a pharmacy long enough, pharmacy legal issues are bound to happen to you. . Types of Pharmacy Legal Issues for Pharmacy Owners. Unfortunately, I have experienced many of these issues myself. Each legal issue is unique. The post Pharmacy Legal Issues first appeared on DiversifyRx.

Pharmaceutical Commerce
APRIL 3, 2024
Click the title above for a link to open the Pharmaceutical Commerce April 2024 issue in an interactive PDF format.

European Pharmaceutical Review
SEPTEMBER 4, 2023
Included in Issue 4 of European Pharmaceutical Review : FOREWORD Dissolution testing – a dual role David Elder, David P Elder Consultancy REGULATORY INSIGHT EU packaging reform: a prescription for change?

European Pharmaceutical Review
APRIL 24, 2023
Included in this issue of European Pharmaceutical Review : FOREWORD New quality requirements for tobacco products David Elder, David P Elder Consultancy QA/QC MASS SPECTROMETRY The benefits of mass spectrometry for expediting biologics to patients Ian Anderson, Mostafa Zarei and Qifeng Zhang, Lonza IN-DEPTH FOCUS: Bioprocessing/Bioproduction Realising (..)

European Pharmaceutical Review
JUNE 27, 2023
Included in this issue of European Pharmaceutical Review : FOREWORD Nitrosamines: the beginning of the end? Elizabethann Wright, Cooley LLP The post <em>European Pharmaceutical Review</em> Issue 3 2023 appeared first on European Pharmaceutical Review.

BioPharm
MAY 17, 2023
The agency’s safety committee is reviewing hydroxyprogesterone medicines and issuing a reminder on safety issues regarding fluoroquinolone antibiotics.
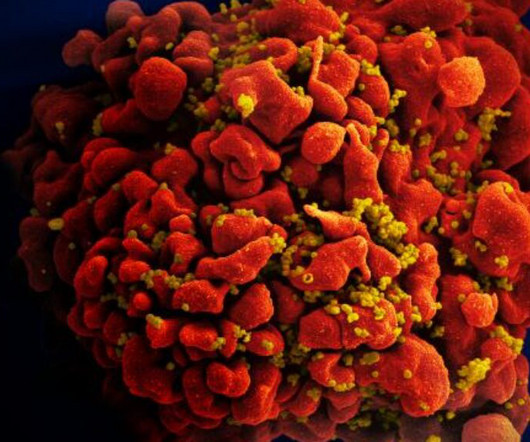
STAT
OCTOBER 3, 2023
In the latest battle over access to medicines, Colombian authorities have decided to issue a compulsory license for a needed HIV treatment, a step that underscores ongoing friction between the pharmaceutical industry and cash-strapped governments around the world. Continue to STAT+ to read the full story…

Pharmacy Times
SEPTEMBER 7, 2023
Christina Madison, PharmD, FCCP, AAHIVP, speaks with Ryan Haumschild, PharmD, MS, MBA, to discuss current issues in oncology, including drug shortages and patient access.

STAT
AUGUST 22, 2023
FDA greenlights device to combat bladder issues Read the rest… You’re reading the web edition of STAT Health Tech, our guide to how tech is transforming the life sciences. Sign up to get this newsletter delivered in your inbox every Tuesday and Thursday.

pharmaphorum
NOVEMBER 2, 2023
Novo Nordisk shrugs off supply issues with record sales Phil.Taylor Thu, 02/11/2023 - 11:17 Bookmark this

Fierce Pharma
JANUARY 9, 2024
But the Japanese company will have to clear up manufacturing issues first. The FDA has sent a complete response letter to Astellas, rejecting zolbetuximab because of unresolved deficiencies identified in a pre-license inspection of a third-party manufacturing facility.

Drug Topics
JANUARY 3, 2023
A look at what’s to come in the January issue of Drug Topics®.

Drug Store News
DECEMBER 14, 2023
Drug Store News held its 25th annual Industry Issues Summit in New York City on Dec. 13, where industry leaders weighed in on a myriad of challenges and opportunities.

STAT
OCTOBER 19, 2023
RNA medicines startup Laronde is merging with another startup in the wake of a scientific misconduct issue that affected its leading drug candidates. Laronde was founded by biotech creation firm Flagship Pioneering in 2017 to develop a new type of genetic medicine called endless RNA.

Drug Topics
DECEMBER 1, 2022
A look at what’s to come in the December issue of Drug Topics®.

European Pharmaceutical Review
AUGUST 24, 2022
Included in this issue: NITROSAMINES. The post European Pharmaceutical Review Issue 4 2022 appeared first on European Pharmaceutical Review. Risks presented by active pharmaceutical ingredient nitrosamines. David Elder, David P Elder Consultancy. ENVIRONMENTAL MONITORING. Hannah Balfour with comment from Mike Russ at Genentech.

Drug Topics
NOVEMBER 1, 2022
A look at what’s to come in the November issue of Drug Topics®.

Pharmaceutical Commerce
JUNE 28, 2023
In an interview with Pharma Commerce Editor, Nicholas Saraceno, Courtney Granville, Director, Scientific Affairs, Drug Information Association, discusses issues impacting broader supply chains.

STAT
DECEMBER 27, 2023
The fundamental issues and intriguing stories that characterized the year gone by will to continue to loom. So once again, we will take a whack at identifying some of the most noteworthy developments to watch. However, if these seem familiar, there is good reason.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?

Let's personalize your content