CMO Moves: Regulatory catalysts for drug manufacturing-April
Pharmaceutical Technology
JULY 14, 2022
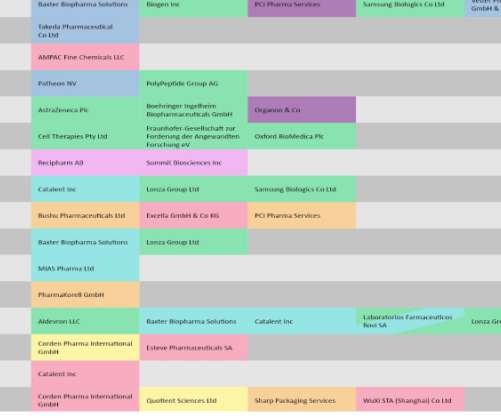
This analysis covers late April to May and is based on a list of CMOs impacted by regulatory decisions by the US Food and Drug Administration (FDA), European Medicines Agency (EMA), and reimbursement authorities like the UK’s National Institute of Health and Care Excellence (NICE). Covid-19 vaccines stay in the spotlight.












Let's personalize your content