FDA Proposes Front-of-Package Nutrition Label for Most Packaged Foods
Pharmacy Times
JANUARY 17, 2025
If finalized, the requirement would include readily available nutrition information, including saturated fat, sodium, and added sugar content.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Pharmacy Times
JANUARY 17, 2025
If finalized, the requirement would include readily available nutrition information, including saturated fat, sodium, and added sugar content.

Pharmaceutical Technology
MAY 16, 2023
The US Food and Drug Administration (FDA) has approved expanded labelling for Cumberland Pharmaceuticals’ Caldolor therapy to include use in infants. Caldolor has already obtained FDA approval for pre-operative administration.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

The FDA Law Blog
APRIL 25, 2023
The primary vehicle for FDA to request cybersecurity information in premarket submissions has been guidance documents. The primary vehicle for FDA to request cybersecurity information in premarket submissions has been guidance documents. We have recently blogged on this topic (“ Is my software a medical device? ”).

The FDA Law Blog
JUNE 26, 2024
Lenz, Principal Medical Device Regulation Expert — FDA recently released a new eSTAR template for device pre-submissions and 513(g) Requests for Information, referred to as PreSTAR. A pre-submission provides the submitter an opportunity to obtain FDA feedback prior to a planned medical device premarket submission.

STAT
AUGUST 20, 2024
And a group of researchers — including two from the FDA’s Center for Drug Evaluation and Research — think artificial intelligence could uncover more signs of these issues, including from electronic health records, social media posts, and clinical databases referencing certain drugs.

The FDA Law Blog
OCTOBER 24, 2023
By Dara Katcher Levy — Yesterday, FDA published a new Draft Guidance, “ Communications from Firms to Health Care Providers Regarding Scientific Information on Unapproved Uses of Approved/Cleared Medical Products Questions and Answers ” (SIUU Guidance or Draft Guidance).
Pharmaceutical Technology
MARCH 22, 2023
As computing has developed in the past decade, so has the potential to store and use information in the cloud. The US Food and Drug Administration (FDA) has provided guidance for the use of RFID in the drug supply chain and to standardise the data format. In recent times, various global administrators have issued regulatory standards.

The FDA Law Blog
APRIL 2, 2024
Koblitz — Every year, federal agencies submit a budget request to Congress to fund various agency initiatives, and every year FDA includes a list of legislative proposals that it would like to see come out of Congress. FDA believes this change would effectuate timelier and more cost-efficient generic drug development.”
The Checkup by Singlecare
NOVEMBER 11, 2024
As a brand-name prescription drug, Lexapro is FDA -approved to treat major depressive disorder and anxiety disorders. Although trazodone is FDA -approved for depression, it’s primarily known for its off-label use as a sleep aid due to its sedative effects.

Pharmaceutical Technology
APRIL 11, 2023
This label expansion makes Orkambi the only disease-modifying CF medication available to patients of this age in Canada. This label expansion follows a similar label change in the US in September 2022. Health Canada is granting this new label expansion based on recent results from a Phase III study.

The Checkup by Singlecare
NOVEMBER 9, 2023
Food and Drug Administration (FDA) just approved Zepbound (tirzepatide) for chronic weight management. The injectable medication is a new version of Eli Lilly’s Mounjaro, which is approved by the FDA to control blood sugar in people with Type 2 diabetes. Zepbound, on the other hand, has been FDA-approved for weight loss.

The FDA Law Blog
JULY 23, 2024
Lenz, Principal Medical Device Regulation Expert — For several years, FDA has requested that sponsors of drug or biologic led combination products identify essential performance requirements (EPRs) related to the device constituent in their applications. By Adrienne R. does not use this term. does not use this term.

European Pharmaceutical Review
JANUARY 25, 2024
Draft guidance published by the US Food and Drug Administration (FDA) in December 2023, discussed quality considerations for topical ophthalmic drug products, including key considerations for extractables and leachables (E&L) testing. Ophthalmic drug products should be evaluated for extractables and leachables, FDA asserted.
The Checkup by Singlecare
DECEMBER 6, 2024
Because clonidine works differently than other blood pressure medications, it also has several off-label uses including anxiety, insomnia, attention deficit hyperactivity disorder (ADHD), and alcohol withdrawal. While clonidine is FDA approved to treat high blood pressure, its not considered a first-line treatment option.

pharmaphorum
AUGUST 21, 2022
Shares in Axsome Therapeutics have rocketed on FDA approval of its depression therapy Auvelity (formerly AXS-05) – a year after its approval was held up by the regulator. However, it has a broader label as unlike J&J’s drug it is indicated for use in previously-untreated MDD. Photo by Sydney Sims on Unsplash.

STAT
MARCH 8, 2024
The Food and Drug Administration asks manufacturers for a list of materials before the media goes on the market, an FDA spokesperson told STAT. Companies will sometimes give clinics this information voluntarily, along with required labeling information like mouse embryo data, instructions for use, and warnings.

BuzzRx
JANUARY 27, 2023
Food and Drug Administration (FDA) has to undergo several rigorous phases before approving new medications. Once the FDA approves a new drug, it means that when using this drug for an approved condition, the potential benefits outweigh the potential risks. The term “on-label use” of a drug may seem unfamiliar to most people.
pharmaphorum
AUGUST 24, 2020
In the United States, the 21st Century Cures Act encouraged the Food and Drug Administration (FDA) to review and communicate patient experience data from trials – but the lack of a common framework for submissions and space on product labels has, until now, been something of a stumbling block. .

Pharmaceutical Technology
JANUARY 24, 2023
The US Food and Drug Administration (FDA) has granted clearance for Neurogene’s investigational new drug (IND) application for NGN-401 to treat Rett syndrome. Neurogene stated that the FDA IND clearance allows it to commence a Phase II/II trial of NGN-401 in female paediatric Rett syndrome patients.

pharmaphorum
OCTOBER 4, 2022
On 27th September 2022, the Food and Drug Administration (FDA) issued its final guidance for industry and FDA staff clinical decision support (CDS) software, which has been anticipated since the Center for Devices and Radiological Health (CDRH) listed the guidance as a top priority for fiscal year 2022. Criteria for regulation.

The FDA Law Blog
JULY 10, 2023
After a firm submits a 510(k) to FDA, FDA will request still more information after a first-pass review. According to the 2 nd Quarter FY2023 MDUFA V Performance Report , FDA issued a request for additional information (AI request) on the first FDA review cycle for 63% to 68% of 510(k)s submitted in FY2018 to FY2022.

IDStewardship
MARCH 12, 2023
Article 9: How to Harness the Power of Social Media for Quality Drug Information in Infectious Diseases: Perspectives on Behalf of the Society of Infectious Diseases Pharmacists Find it here. This articles and the previous editions are great sources of information for what is happening in antimicrobial stewardship.

The Checkup by Singlecare
DECEMBER 2, 2024
Pharmaceutical options such as benzodiazepines like diazepam and antidepressants like trazodone are sometimes used off-label to treat insomnia in adults. Food & Drug Administration (FDA) to treat generalized anxiety disorder. Previously sold under the brand name BuSpar, its main purpose is to alleviate symptoms of anxiety.
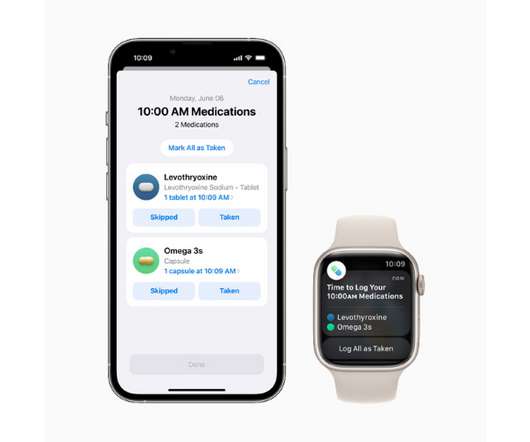
pharmaphorum
JUNE 8, 2022
The new tool works as a component of Apple’s Health app and will let users add drugs or other health products like vitamins and supplements to a personal list – either by scanning a label or finding the product in a directory – and create custom schedules for them. New atrial fibrillation feature.

The Checkup by Singlecare
MARCH 18, 2025
Semaglutide is the active ingredient in two brand-name injectable drugs approved by the Food and Drug Administration (FDA). Ozempic is FDA approved for blood sugar control in people with Type 2 diabetes , as well as cardiovascular risk reduction in people with Type 2 diabetes and heart disease. What is tirzepatide?

The FDA Law Blog
JUNE 27, 2023
Walsh — Last fall, we blogged about the process FDA uses to review allegations of regulatory misconduct against device manufacturers, suggesting greater transparency on the FDA process was needed (see here ). FDA also requests a “detailed description of the allegation with any available supporting documentation.”

The Checkup by Singlecare
DECEMBER 30, 2024
Additionally, gabapentin is typically used as a human medicine; its prescribed off-label for dogs since it doesnt have FDA approval for them yet. Both vets urge dog owners to keep the medication out of reach of the petlike in a locked cabinet, for exampleand to follow the prescription label carefully.

The FDA Law Blog
FEBRUARY 21, 2024
Mullen — More than five years after FDA first announced its plan to harmonize 21 CFR Part 820 with ISO 13485, on February 2, 2024, FDA finally issued the Quality Management System Regulation (QMSR) Final Rule. FDA further retained some definitions in the QSMR. Notably, Part 820 will look different. Revised § 820.3

The FDA Law Blog
SEPTEMBER 29, 2022
As readers may recall, the current regulation provides for four options for a disclosure statement, i.e., text disclosure, symbol disclosure, electronic disclosure (QR code with a phone number for further information), and a text message disclosure.

The Checkup by Singlecare
MARCH 4, 2025
Adults and pediatric patients 12 years and older Adults 17 years and older Qsymia vs. phentermine: Conditions treated Qsymia is approved by the Food and Drug Administration (FDA) for weight loss and long-term weight management in adults and pediatric patients 12 years and older with obesity. of body weight compared to 13.7%

Digital Pharmacist
OCTOBER 24, 2022
The FDA protects public health by ensuring the efficacy and safety of human and veterinary drugs, including biological products and medical devices. It is critical for the FDA to continue to develop new advances in therapies to aid our communities in order to achieve better lives through better health. Date of Approval: 9/30/2022.

STAT
SEPTEMBER 13, 2024
One doctor said he thinks the company wants to discourage using the drugs for unapproved purposes, a practice called off-label prescribing, which is legal and accepted in American medicine. As with company drug advertisements, social-media posts sponsored by drug makers are required by the FDA to cite the risks.

The FDA Law Blog
AUGUST 13, 2023
The information is presented in a Q&A format. In the case of contract manufacturers, either the contract manufacturer or the person whose name appears on the label (i.e., In addition to the mandatory information to be submitted (i.e., In addition to the mandatory information to be submitted (i.e.,

Pharma Marketing Network
MARCH 8, 2025
Pay-per-click (PPC) advertising allows brands to reach healthcare professionals (HCPs) and patients when they are actively searching for treatment options, drug information, or medical education. Optimize landing pages to ensure message consistency and compliance with FDA regulations. What platforms are best for pharma PPC campaigns?

European Pharmaceutical Review
SEPTEMBER 27, 2022
The US Food and Drug Administration (FDA) have published initial guidance deliberating how ethical it is for children to take part in research trials. Thus, numerous drugs and medical devices approved and licensed by the FDA are missing essential paediatric-specific information on their product labels.

The FDA Law Blog
OCTOBER 24, 2022
These data are routinely collected from a variety of sources, such as electronic health records, providing information on health and healthcare in actual patients, rather than in the controlled environment of a clinical trial. The sponsor and FDA reach agreement on the study design information to be publicly disclosed.

The Checkup by Singlecare
MAY 13, 2025
It is FDA approved to help manage symptoms of asthma and improve lung function in adults and pediatric patients five years of age and older. Dulera is also prescribed off-label to help with breathing problems in adults with chronic obstructive pulmonary disorder (COPD). The post Is Dulera a steroid? appeared first on The Checkup.

The Checkup by Singlecare
DECEMBER 12, 2024
Ozempic is also sometimes prescribed off-label (for a non-FDA-approved use) as a weight loss drug. An overview of semaglutide Novo Nordisk, the pharmaceutical company that manufactures FDA-approved semaglutide products, currently manufactures three medicines that contain semaglutide. This is the case with semaglutide.

The Checkup by Singlecare
APRIL 17, 2025
Food and Drug Administration (FDA) for chronic weight management in adults with a starting, or baseline , body mass index ( BMI ) of 30 or more ( obesity ). You can contact your specific plan for more information about weight loss drug coverage , and specifically, Saxenda. It is approved by the U.S.

The FDA Law Blog
MAY 8, 2023
Javitt — FDA recently published a long-awaited draft guidance aimed at reducing the need for prior FDA authorization of modifications to artificial intelligence/machine learning (AI/ML)-enabled device software functions (ML-DSFs). Baumhardt, Senior Medical Device Regulation Expert & Philip Won & Gail H. See 21 CFR 807.81(a)(3)

The FDA Law Blog
NOVEMBER 29, 2023
Since that time, FDA issued a draft guidance for predetermined change control plans (PCCPs) for Artificial Intelligence/Machine Learning (AI/ML) software functions. FDA considers these guiding principles as complimentary to their recent efforts around PCCPs including their proposed draft guidance on PCCPs.

The Checkup by Singlecare
JULY 8, 2024
It’s also relatively new: Vraylar came on the market in 2015, and the Food and Drug Administration (FDA) has been slowly approving it for different uses since then, including a 2022 approval for use alongside antidepressants in people with major depressive disorder. Can you take Vraylar while pregnant?

The FDA Law Blog
SEPTEMBER 22, 2024
The webinar largely consisted of summarizing the general requirements under Parts 803, 806 and 820.198, which we do not reproduce here ( but see another of our prior blog posts discussing these requirements and their applicable to LDTs in greater detail; you can also find FDA’s slides from the webinar here ).

The FDA Law Blog
APRIL 25, 2024
This Revised Draft Guidance provides considerations for manufacturers, packers or distributors (dubbed “firms”) of prescription biological reference products, biosimilar products, and interchangeable biosimilar products presenting data and information about such products in promotional materials in a truthful and non-misleading way.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content