Advanced Therapy and Advanced Manufacturing for Unmet Medical Needs
ISPE
SEPTEMBER 7, 2023
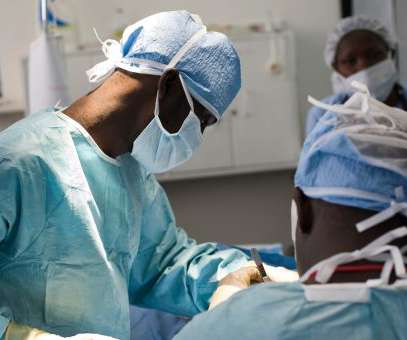
The emergence of new therapies leads to the need for multi-modal manufacturing facilities. supply chain management, pharmaceutical quality system, and so on). This encompasses process development, facility design, equipment selection, quality system establishment, supply chain management, as well as personnel training.












































Let's personalize your content