Convenience an Important Factor in the Multiple Myeloma Treatment Equation
Drug Topics
JANUARY 2, 2023
The COVID-19 pandemic revealed the advantages of treatment that involved fewer in-person office visits, according to recent research.
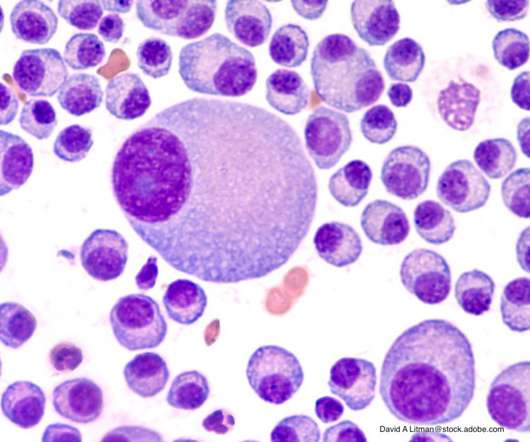
Drug Topics
JANUARY 2, 2023
The COVID-19 pandemic revealed the advantages of treatment that involved fewer in-person office visits, according to recent research.
Pharmacy Times
JANUARY 2, 2023
Pharmacists should educate patients about managing respiratory syncytial virus and let them know they may soon be able to get vaccinated.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Drug Topics
JANUARY 2, 2023
Take a look back at our most popular women's health coverage for 2022.

Fierce Healthcare
JANUARY 2, 2023
Biden signs $1.7T omnibus, paving way for multiple health policy priorities. pminemyer. Mon, 01/02/2023 - 22:24.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven
Board Vitals - Pharmacist
JANUARY 2, 2023
The Internal Medicine Board Certification Exam is the final step for doctors looking to specialize in Internal Medicine. This in-depth exam covers a range of different topics, including Hematology, Psychiatry, and Dermatology. Passing the boards is not a guarantee, but with the right plan in place, you will be confident and prepared come test day. What is the Internal Medicine Board Exam?

Pharma Tutor
JANUARY 2, 2023
Computational Analysis of HCV Entry Inhibitors for Hepatitis C Treatment – A Molecular Docking Approach. admin. Mon, 01/02/2023 - 20:35. About Author: Karthika M. Postgraduate in Bioinformatics. Bharathiar University m.karthika1@gmail.com.
Pharmacy Technician Pulse brings together the best content for pharmacy technicians from the widest variety of industry thought leaders.

The FDA Law Blog
JANUARY 2, 2023
Hosted by American Conference Institute, the FDA Boot Camp returns for its 40th iteration with the continued intent of providing an essential working knowledge of core FDA concepts, and real-world examples that will help you to excel in your everyday practices. This year’s conference co-chairs include Stacy Cline Amin (Partner, Morrison Forester) and Kurt R.

Pharmaceutical Technology
JANUARY 2, 2023
South Korean biotechnology company Alteogen has signed an exclusive license agreement with Swiss company Sandoz to develop and market biosimilar products that are enabled by the former’s Hybrozyme technology. Under the terms of the agreement, Sandoz will have the global rights to use Alteogen’s novel hyaluronidase, ALT-B4, for the development and commercialisation of a subcutaneous version of a biosimilar product.

Oswald's
JANUARY 2, 2023
January 1st, 2023 marks the beginning of my third year running the ol’ family pharmacy here in Naperville. I’m grateful for our wonderful team (pictured on the right) that continues to care for our community in our 148th year of business. Thanks to our Operations Manager Karen for creating the wonderful collage of our team… The post A Message From Alex January 2023 appeared first on Oswald's Pharmacy.

Pharmaceutical Commerce
JANUARY 2, 2023
A Q&A with AstraZeneca’s Country President for Belgium & Luxembourg, Keira Driansky.

Speaker: Chris Antypas and Josh Halladay
Access to limited distribution drugs and payer contracts are key to pharmacy expansion. But how do you prepare your operations to take the next step? Meaningful data: Collect and share clinical data regarding outcomes, utilization, and more Reporting: Limited distribution models require efficient tracking and reporting systems Workflows: Align workflows with specific pharma and payer contractual requirements For in-depth, expert insights on pharmacy expansion, watch this webinar from Inovalon.

Oswald's
JANUARY 2, 2023
The post January 2023 Promotions appeared first on Oswald's Pharmacy.
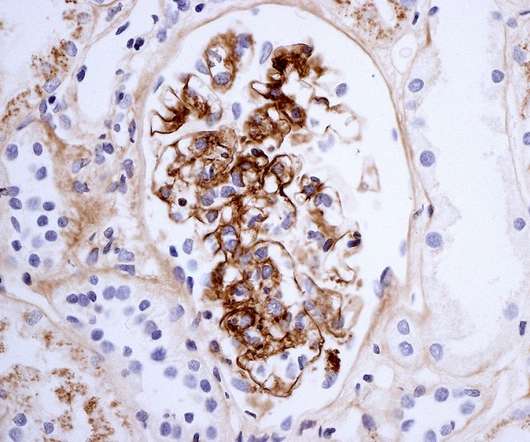
Pharmaceutical Technology
JANUARY 2, 2023
The China National Medical Products Administration’s (NMPA) Center for Drug Evaluation (CDE) has recommended priority review for Everest Medicines’ new drug application (NDA) of Nefecon to treat primary immunoglobulin A nephropathy (IgAN). The regulatory support for the accelerated approval of Nefecon is to treat IgAN in adult patients who are at risk of rapid disease progression.

Fierce Pharma
JANUARY 2, 2023
Mayo spreads support to InSitu with prolonged-release cancer drug co-development pact. ntaylor. Tue, 01/03/2023 - 01:53.

Pharmaceutical Technology
JANUARY 2, 2023
Danicamtiv is under clinical development by Bristol-Myers Squibb and currently in the Phase I and Phase II in clinical pathway. The characteristics of the clinical trial as well as other attributes related to the drug, regulations, and company play a fundamental role in ensuring the likelihood of transition that the drug moves from its current development stage to next.

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.

Pharmacy Times
JANUARY 2, 2023
When it comes to vaccination efforts, remember that patients 65 years or older are not a homogeneous group.

Pharmaceutical Technology
JANUARY 2, 2023
Lifirafenib maleate is under clinical development by BeiGene and currently in the Phase II in clinical pathway. The characteristics of the clinical trial as well as other attributes related to the drug, regulations, and company play a fundamental role in ensuring the likelihood of transition that the drug moves from its current development stage to next.

Pharmaceutical Technology
JANUARY 2, 2023
BI-1361849 is under clinical development by Boehringer Ingelheim International and currently in the Phase II in clinical pathway. The characteristics of the clinical trial as well as other attributes related to the drug, regulations, and company play a fundamental role in ensuring the likelihood of transition that the drug moves from its current development stage to next.
Let's personalize your content