Why a 'fundamental shift' in regulatory submissions is on the way
PharmaVoice
JULY 11, 2022
Deloitte's senior manager of R&D and regulatory practices explains how technology and strategy must converge to smooth out the bulky drug approval process.

PharmaVoice
JULY 11, 2022
Deloitte's senior manager of R&D and regulatory practices explains how technology and strategy must converge to smooth out the bulky drug approval process.
Pharmacy Times
JULY 11, 2022
The non-estrogen pill was first approved by the FDA in 1973.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

PharmaVoice
JULY 11, 2022
Phil Brown, Dermavant’s chief medical officer, discusses VTAMA — the first topical cream approved for psoriasis in 25 years.
Pharmacy Times
JULY 11, 2022
Christopher Fine, MD, FACC, a cardiologist at National Jewish Health, discusses how pharmacists can support patients with cancer who have a much higher risk of heart disease.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven
European Pharmaceutical Review
JULY 11, 2022
The novel coronavirus vaccine has been developed at Caltech and The University of Oxfor d. Funding of up to $30 million will support vaccine design, its development through Phase I trials and regulatory activities. . Manufacturing efforts for the project will be led by the UK’s Centre for Process Innovation ( CPI ) using microbes engineered by biotechnology Ingenza Ltd.

Pharmacy Times
JULY 11, 2022
There is new guidance needed for pediatric immunizations, and pharmacists should be prepared for what's to come.
Pharmacy Technician Pulse brings together the best content for pharmacy technicians from the widest variety of industry thought leaders.

Pharmacy Times
JULY 11, 2022
Lifestyle modifications are the cornerstone of treatment.
pharmaphorum
JULY 11, 2022
Researchers in the US have developed an app that uses a smartphone camera and artificial intelligence algorithms to assess images of patients’ poo for signs of disorders like irritable bowel syndrome (IBS). The tool is intended as an alternative to patients’ self-reporting stool form and frequency using the seven-point Bristol Stool Scale (BSS), which ranks consistency from hard to liquid but can produce highly variable results.

Pharmacy Times
JULY 11, 2022
Health Mart also presented the first ever Innovation Award at McKesson ideaShare 2022.
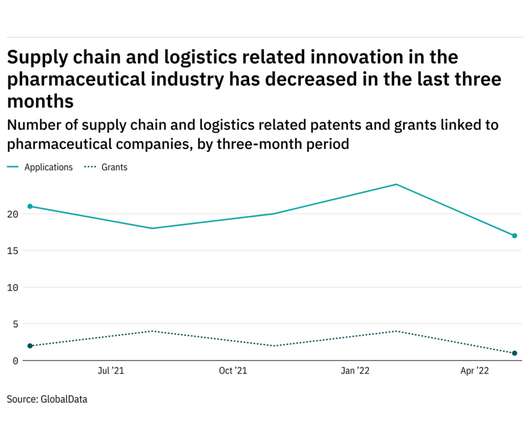
Pharmaceutical Technology
JULY 11, 2022
Research and innovation in supply chain & logistics in the pharmaceutical sector has declined in the last year. The most recent figures show that the number of supply chain and logistics related patent applications in the industry stood at 17 in the three months ending May - down from 21 over the same period in 2021. Figures for patent grants related to supply chain and logistics followed a similar pattern to filings - shrinking from 2 in the three months ending May 2021 to 1 in the same per

Speaker: Chris Antypas and Josh Halladay
Access to limited distribution drugs and payer contracts are key to pharmacy expansion. But how do you prepare your operations to take the next step? Meaningful data: Collect and share clinical data regarding outcomes, utilization, and more Reporting: Limited distribution models require efficient tracking and reporting systems Workflows: Align workflows with specific pharma and payer contractual requirements For in-depth, expert insights on pharmacy expansion, watch this webinar from Inovalon.

Pharmacy Times
JULY 11, 2022
Learning the Pharmacists' Patient Care Process steps and being mindful or internalizing those can help set best practices for pharmacy team members.

PharmExec
JULY 11, 2022
Kobel discusses taking a company public during a challenging time in the market.

Pharmacy Times
JULY 11, 2022
Patients with refractory chronic migraine experience short- and medium-term pain relief and minimal adverse drug effects from lidocaine infusions.

PharmExec
JULY 11, 2022
Generating an actual brainstorm is elusive. Every marketer, strategist, creative person in every industry has probably seen far more of the opposite.

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.
Pharmacy Times
JULY 11, 2022
Subcutaneous immunotherapy-induced antibodies may serve as a potential allergen-targeted biologics candidate for the treatment of allergic asthma.

pharmaphorum
JULY 11, 2022
Vertex has doubled down on cell-based therapies for type 1 diabetes (T1D), buying ViaCyte in a $320 million deal that it says will accelerate development of its own candidate VX-880. The deal has been announced just a few days after the FDA lifted a clinical hold on a phase 1/2 trial of VX-880 – a stem cell therapy Vertex acquired through its near $1 billion acquisition of Semma in 2019 – because the FDA wanted to see additional data before allowing the dose to be increased.

Pharmacy Times
JULY 11, 2022
Emphasizing the accessible nature of pharmacies and why this is essential for patients can help legislators and others understand why changes are necessary.

Pharmaceutical Technology
JULY 11, 2022
Innoviva has signed a definitive merger agreement for the acquisition of all of the outstanding shares of La Jolla Pharmaceutical Company for $6.23 for each share in cash or an enterprise value of nearly $149m. As per the deal, Innoviva will acquire La Jolla through its wholly-owned subsidiary. La Jolla focuses on marketing innovative treatments that can enhance outcomes in patients with life-threatening diseases.

Advertisement
Are you still using workarounds to manage your daily operations? To achieve peak performance, it's time to explore other options for specialty and infusion pharmacy software. Streamline pharmacy operations and improve clinical performance with automated processing, real-time data exchange, and electronic decision support. Download this helpful infographic to: Drive efficiency and patient adherence from referral receipt to delivery and ongoing care – all with our Pharmacy Cloud.

Pharmacy Times
JULY 11, 2022
Joe Duarte, co-founder and president of ContinuumMD, discusses a program model that allows pharmacies to provide virtual and in-person primary care options for rural communities with health care access challenges.
Pharmaceutical Technology
JULY 11, 2022
The US Food and Drug Administration (FDA) has granted approval for the supplemental Biologics License Application (sBLA) of Horizon Therapeutics for the Krystexxa (pegloticase) injection, given along with methotrexate, to help uncontrolled gout patients attain a complete response to treatment. The label expansion for Krystexxa plus methotrexate is based on the data from the randomised, controlled MIRROR clinical trial in adults with the condition.

Pharmacy Times
JULY 11, 2022
Sinex helps to relieve sinus congestion and pressure.

NF2 BioSolutions
JULY 11, 2022
When I left school I studied child development, I met with a careers advisor who told me to be a nurse I’d need to go to university. Straight away I said I wasn’t good enough for uni & that was that. Fast forward 15 years & NF2 showed me, my strength & resilience & I decided life was too short not to at least try. Turns out I was good enough, after gaining a 1st class degree, I stepped into my 1st nursing job at Addenbrookes hospital in September 2020 as a Neuro Nurse
Pharmacy Times
JULY 11, 2022
Ofatumumab (Kesimpta) is a CD20-directed cytolytic antibody indicated for the treatment of relapsing forms of multiple sclerosis.

OctariusRx
JULY 11, 2022
Preview the new medications of 2022, as we reach the halfway point of the year. The U.S. Food and Drug Administration (FDA) is constantly reviewing and approving new medications and the number of medications approved in recent years, has drastically increased. Keeping up with all the changes can be a full-time job. In this post, we look at the most recent approvals to keep you informed and help you keep your patients safe.

Pharmacy Times
JULY 11, 2022
Harnessing the current momentum and engaging with legislators is crucial for the pharmacy industry to grow and expand.

Colors Make me Happy
JULY 11, 2022
During the winter break of 2021, I decided to pack my stuff and head for the deep Colombian Amazon Forest. I had been thinking about spending time with the indigenous people of Amazonia for a while and after two years of social isolation, stress, and turmoil, I felt the right time had come. My plane landed in Leticia, the capital of the Amazonas and the southernmost town in Colombia.

Pharmacy Times
JULY 11, 2022
Cole McCoy, PharmD, and Danielle Marcotulli, APN, RN, MSN, FNP-BC, AOCNP explain the additional agents being studied as maintenance therapy options.

PharmExec
JULY 11, 2022
Pharm Exec chats with Read Roberts about finding the right DOLs in a crowded social media landscape.

Pharmacy Times
JULY 11, 2022
James McCloskey, MD, analyzes result data from the QUAZAR AML-001 trial that focused on oral azacytidine.

PharmExec
JULY 11, 2022
Kenah joins the leadership team with 18 years of program management, marketing, and customer engagement experience in the healthcare industry, including for WebMD and SonderMind.

Pharmaceutical Commerce
JULY 11, 2022
Vendor relationships are best supported by practices that emphasize efficiency, visibility, and the streamlining of the invoice to pay process.

PharmExec
JULY 11, 2022
InnoVision Español will serve businesses and brands wanting to reach the Hispanic community and Hispanic-oriented clients and companies.

Pharmaceutical Commerce
JULY 11, 2022
A comprehensive data and analytics strategy is now table stakes for pharmaceutical and life science organizations, writes Simon Andrews.
Let's personalize your content