Lantidra Cell Therapy for Type 1 Diabetes Approved by FDA
Drug Topics
JUNE 29, 2023
The approval was based on safety and efficacy data from 30 participants in 2 open-label, single-arm studies that received 1 to 3 infusions of donislecel.

Drug Topics
JUNE 29, 2023
The approval was based on safety and efficacy data from 30 participants in 2 open-label, single-arm studies that received 1 to 3 infusions of donislecel.

PharmaVoice
JUNE 29, 2023
From AI to diversity and decentralized trials, here’s how clinical trial operations are evolving.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Drug Topics
JUNE 29, 2023
The increase is expected to be somewhat offset by the impact of biosimilars coming to market and more care being provided in outpatient settings.

PharmaVoice
JUNE 29, 2023
Transgender and nonbinary patients suffer more adverse health outcomes than the general population, yet are often underrepresented in clinical research. Now, there’s momentum to change the status quo.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Drug Topics
JUNE 29, 2023
Although biosimilars have been introduced with the aim of competing with expensive biologic therapies, their adoption has been more gradual than expected.

Pharmacy Times
JUNE 29, 2023
Yet, physicians and advanced practice practitioners may have a limited understanding of the scope of an ambulatory pharmacy clinic and the value a clinical pharmacist can provide.
Pharmacy Technician Pulse brings together the best content for pharmacy technicians from the widest variety of industry thought leaders.

Fierce Pharma
JUNE 29, 2023
After an initial rejection in 2020 and a review delay earlier this year, BioMarin’s Roctavian has finally got the FDA go-ahead to introduce a gene therapy for a not-so-rare disorder. | After an initial rejection in 2020 and a review delay earlier this year, BioMarin’s Roctavian has finally got the FDA go-ahead to introduce a gene therapy for a not-so-rare disorder.
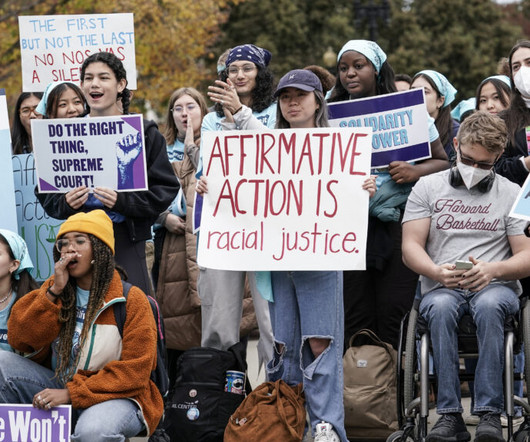
STAT
JUNE 29, 2023
Overturning decades of precedent, the U.S. Supreme Court on Thursday struck down the use of affirmative action, ruling that it is unconstitutional for colleges, universities — and professional schools for law, medicine, and nursing — to consider race as one factor in deciding who they will admit. The decision comes as a blow to many in the field of medicine, which has been unable to appreciably increase the numbers of Black, Hispanic, and Indigenous doctors in recent decades.
Pharmacy Times
JUNE 29, 2023
The impact of pharmacist on reducing medication expenses cannot be overstated as they leverage their medication management expertise within hospital settings through collaboration with the 340B program.
pharmaphorum
JUNE 29, 2023
FDA approves first cell therapy for type 1 diabetes Phil.

Speaker: Chris Antypas and Josh Halladay
Access to limited distribution drugs and payer contracts are key to pharmacy expansion. But how do you prepare your operations to take the next step? Meaningful data: Collect and share clinical data regarding outcomes, utilization, and more Reporting: Limited distribution models require efficient tracking and reporting systems Workflows: Align workflows with specific pharma and payer contractual requirements For in-depth, expert insights on pharmacy expansion, watch this webinar from Inovalon.
Pharmacy Times
JUNE 29, 2023
Investigators found that the first biosimilar of trastuzumab, trastuzumab-anns (Kanjinti; Amgen), did maintain a strong and persistent advantage over the other 4 biosimilar entrants.

pharmaphorum
JUNE 29, 2023
England turns to digital health checks to cut GP pressure Phil.
Pharmacy Times
JUNE 29, 2023
Investigators call for further public awareness of other breast cancer risk factors, besides dense breasts, which could help identify more women who are at high risk.

Fierce Healthcare
JUNE 29, 2023
Value-based care evolved from a pipe dream to a reimbursement code. The “food is medicine” movement is materializing from a pie in the sky into a pie on the table. | As the industry pursues value-based care and payment models, health systems, hospitals, providers and startups are exploring interventions and programs that a generation ago would have not been considered "healthcare.

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.

Pharmacy Times
JUNE 29, 2023
Somatrogon-ghla (Ngenla; Pfizer Inc, OPKO Health Inc) is a subcutaneous injection that is administered once weekly via a device allowing for titration based on patients’ needs.

STAT
JUNE 29, 2023
The ventilator alarm woke me at 3 a.m. It was the fourth alarm in the past two hours. My 5-year-old daughter Molly had been there eight days, intubated and heavily sedated so she wouldn’t feel her skin blistering off her body, the terrible effect of a severe drug reaction. I looked over and saw her nurse, Kelli, holding Molly’s hand with her right hand and trying to chart with her left.

Fierce Healthcare
JUNE 29, 2023
Iodine Software is charging ahead to integrate generative AI technology into its software to make a "quantum leap" in automation and predictive analytics capabilities. | Iodine Software is charging ahead to integrate generative AI technology into its software to make a "quantum leap" in automation and predictive analytics capabilities.

Fierce Pharma
JUNE 29, 2023
Much attention has been paid to Vertex’s efforts to develop a stem cell therapy for Type 1 diabetes. | Much attention has been paid to Vertex’s efforts to develop a stem-cell therapy for Type 1 diabetes. But flying under the radar with an allogenic (donor) gene therapy for the disorder has been Chicago startup CellTrans. Thursday, the FDA signed off on CellTrans’ Lantidra (donislecel), the first cell therapy for type 1 diabetes.

Advertisement
Are you still using workarounds to manage your daily operations? To achieve peak performance, it's time to explore other options for specialty and infusion pharmacy software. Streamline pharmacy operations and improve clinical performance with automated processing, real-time data exchange, and electronic decision support. Download this helpful infographic to: Drive efficiency and patient adherence from referral receipt to delivery and ongoing care – all with our Pharmacy Cloud.

Fierce Healthcare
JUNE 29, 2023
Following the COVID-19 pandemic, the rising tide of mental health concerns—particularly among children and adolescents—has been a major focus in the industry. | Following the COVID-19 pandemic, the rising tide of mental health concerns—particularly among children and adolescents—has been a major focus in the industry.

pharmaphorum
JUNE 29, 2023
Why the key to success lies in leadership diversity Mike.

Fierce Healthcare
JUNE 29, 2023
Medicare Advantage (MA) beneficiaries have less choice when it comes to finding a psychiatrist than Medicaid enrollees or those who buy coverage on the Affordable Care Act (ACA) marketplace do, acc | Medicare Advantage continues to grow, and so does the need for beneficiaries to have access to psychiatrists, says a study in Health Affairs.
Pharmaceutical Technology
JUNE 29, 2023
Genprex has announced that the FDA has granted fast-track designation for Reqorsa, its small cell lung cancer (SCLC) therapy.

Fierce Healthcare
JUNE 29, 2023
Healthcare costs will balloon 7% next year, as payers hammer out new contracts with providers, the labor shortage stretches on and drug prices continue to increase, according to a new

STAT
JUNE 29, 2023
Patients fast before surgery to prevent food from getting into their lungs while they’re under — a serious concern that can lead to lung infection. But for those taking a class of treatments that include the widely popular Ozempic and Wegovy, fasting may not be enough to ensure an empty stomach. The American Society of Anesthesiologists issued guidance Thursday recommending that patients stop taking this class of treatments, called GLP-1 drugs, before undergoing surgery.

pharmaphorum
JUNE 29, 2023
BioMarin’s haemophilia A gene therapy gets US approval Phil.

STAT
JUNE 29, 2023
Rochelle Walensky, the outgoing director of the Centers for Disease Control and Prevention, will miss aspects of leading the nation’s top public health agency when her term ends Friday. But testifying before Congress is not likely to be among them. Walensky squared off against congressional committees 17 times during her 2½ years as head of the CDC, most recently in mid-June, when she faced a grilling from Republicans on the House Committee on Oversight and Accountability.

pharmaphorum
JUNE 29, 2023
Sanofi sets out plan to lead the RSV vaccine market Phil.

STAT
JUNE 29, 2023
DENVER — He could have been a rock star, a religious icon, the way ecstatic applause from thousands of attendees greeted the man dressed in a crisp, all-white suit as he strode onto a backlit stage. He was neither. This was Rick Doblin, the founder and evangelist of a movement to legalize psychedelic MDMA and bring the drug into mainstream medicine.

Pharmaceutical Technology
JUNE 29, 2023
The FDA has announced that Lantidra, a cellular therapy for type 1 diabetes, has become the first treatment of its kind to be approved.
STAT
JUNE 29, 2023
The Food and Drug Administration on Thursday approved a gene therapy to treat people with hemophilia A, an inherited and rare bleeding disorder. The treatment, called Roctavian, is made by BioMarin Pharmaceutical. It was first approved in Europe in August 2022. Roctavian is a one-time therapy that, in clinical trials, dramatically reduced bleeding episodes and helped patients live without the blood transfusions used to treat the disease.

Pharmaceutical Technology
JUNE 29, 2023
Carrie Harney, the vice president of government and regulatory Affairs at US Pharmacopeia (USP) explains US drug shortages.
STAT
JUNE 29, 2023
In 2005, Nick Voyles was diagnosed with hepatitis C after being released from five years of incarceration. A nurse told him he had only six months to live. He was prescribed a drug cocktail, a combination of interferon and ribavirin, that proved ineffective and gave him severe side effects. “It ripped me apart — the entire treatment was killing me,” Voyles, who lives in Bloomington, Ind., recalled.
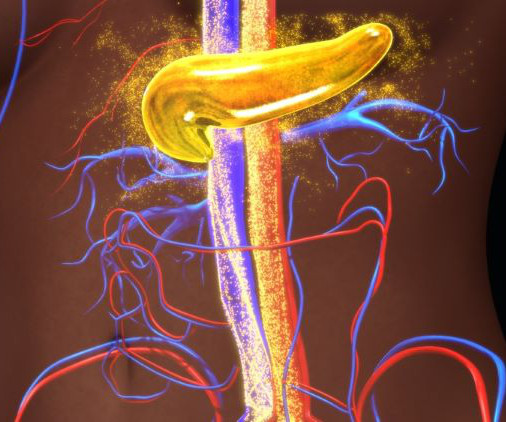
European Pharmaceutical Review
JUNE 29, 2023
The US Food and Drug Administration (FDA) has approved Lantidra, the first cell therapy for certain adults with Type 1 diabetes. The allogeneic pancreatic islet therapy is made from deceased donor pancreatic cells and is indicated for Type 1 diabetes patients who are unable to approach target glycated haemoglobin due to current repeated severe hypoglycaemia episodes even with intensive diabetes management and education.
Let's personalize your content