Problematic Opioid Prescribing Among Patients with Chronic Pain
Drug Topics
AUGUST 8, 2024
Researchers conducted a systematic review of literature exploring the misuse of opioid prescriptions for patients with chronic non-cancer pain.
Drug Topics
AUGUST 8, 2024
Researchers conducted a systematic review of literature exploring the misuse of opioid prescriptions for patients with chronic non-cancer pain.

PharmaVoice
AUGUST 8, 2024
The top drugs at each of the world’s most valuable pharma companies tell the story of just how much the industry has changed over the course of a few years.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Drug Topics
AUGUST 8, 2024
Sam Clark, MD, PhD, founder and CEO of Terran Biosciences, discusses the implications of a potential FDA approval of MDMA-assisted therapy for PTSD.
STAT
AUGUST 8, 2024
Where you live has a relationship to your odds of getting cancer and surviving cancer. Epidemiologists studying this link they see in the data have focused on so-called social determinants of health — poor access to transportation, for example, could make it harder for residents to see a doctor. Places lacking grocery stores with fresh food could mean worse nutrition for locals.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Drug Topics
AUGUST 8, 2024
Researchers addressed the difference between prescribing guselkumab doses every 16 weeks and every 8 weeks for super responder patients with moderate to severe psoriasis.
Pharmacy Times
AUGUST 8, 2024
The injection adds an additional option to address emergency known or suspected opioid overdoses.
Pharmacy Technician Pulse brings together the best content for pharmacy technicians from the widest variety of industry thought leaders.
Fierce Pharma
AUGUST 8, 2024
Following the commercial launch of Bavarian Nordic’s smallpox/mpox vaccine Jynneos earlier this year, the Danish company has locked up yet another supply agreement with the U.S. government. | The Biomedical Advanced Research and Development Authority (BARDA) is handing Bavarian Nordic $156.8 million to “partly replenish” Jynneos vaccine stocks in response to the 2022 outbreak of mpox, the disease formerly known as monkeypox.

Drug Topics
AUGUST 8, 2024
With the high demand in GLP-1 medications, pharmacists are now living in an unprecedented time of filling prescriptions for high-cost brand-name drugs.

Fierce Healthcare
AUGUST 8, 2024
The Department of Health and Human Services’ technology office dropped a new proposed rule on Thursday that would require healthcare entities that contract with HHS to use government-cert | The newly rebranded Office of the Assistant Secretary of Technology Policy released a proposed rule Thursday that could expand the use of government-certified health technology.

Drug Topics
AUGUST 8, 2024
Nalmefene injection is an opioid receptor antagonist and comes in a single-dose, pre-filled auto-injector that delivers 1.5 mg of the medication per actuation.

Speaker: Chris Antypas and Josh Halladay
Access to limited distribution drugs and payer contracts are key to pharmacy expansion. But how do you prepare your operations to take the next step? Meaningful data: Collect and share clinical data regarding outcomes, utilization, and more Reporting: Limited distribution models require efficient tracking and reporting systems Workflows: Align workflows with specific pharma and payer contractual requirements For in-depth, expert insights on pharmacy expansion, watch this webinar from Inovalon.
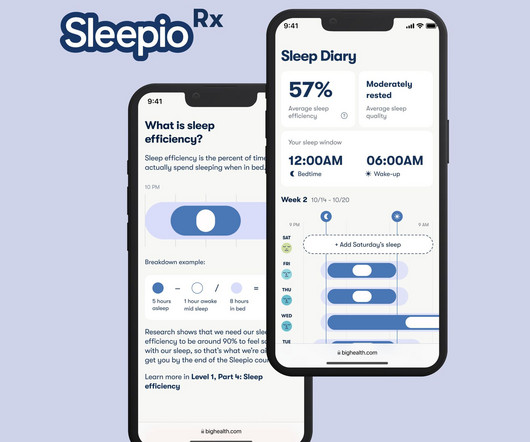
Fierce Healthcare
AUGUST 8, 2024
Big Health, a maker of digital treatments for mental health, has clinched Food & Drug Administration (FDA) clearance of its product for insomnia, Fierce Healthcare has learned in an a | SleepioRx, its flagship digital therapeutic, is intended for the treatment of chronic insomnia and insomnia disorder as an adjunct to usual care in adults over 18.
Pharmacy Times
AUGUST 8, 2024
Isatuximab (Sarclisa; Sanofi) in combination with standard-of-care significantly improved progression-free survival in patients with newly diagnosed multiple myeloma.
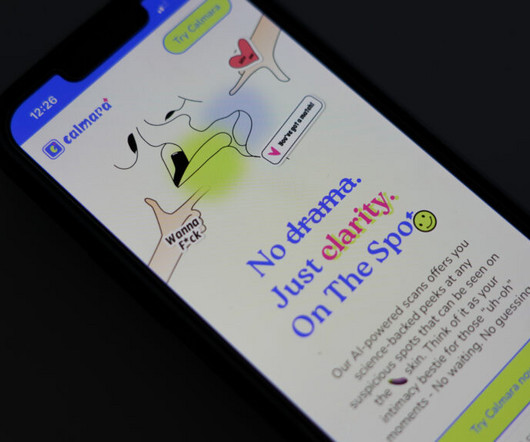
STAT
AUGUST 8, 2024
The company that claimed its AI model could identify sexually transmitted infections from a single penis picture was shut down by the Federal Trade Commission in July. STAT wrote about the app, Calmara, and the immediate critical response it drew from patient advocates, doctors, and privacy experts in April. The founders did not adequately explain how they would protect minors from accessing the app and sensitive user information.

Pharmacy Times
AUGUST 8, 2024
The primary end point of clinical remission at week 26 was met, with 36.7% of individuals treated with RHB-104 achieving clinical remission.

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.

Fierce Healthcare
AUGUST 8, 2024
Mayo Clinic is partnering with startup SandboxAQ to study a new medical device that uses quantum sensing technology and advanced AI algorithms for rapid diagnosis in cardiac care, such as potential | Mayo Clinic is partnering with startup SandboxAQ to study a new medical device that uses quantum sensing technology and advanced AI algorithms for rapid diagnosis in cardiac care, such as detecting a heart attack.

Pharmacy Times
AUGUST 8, 2024
Intravenous Immunoglobulin as a therapy for patients with pyoderma gangrenosum could open new treatment avenues for the rare and challenging condition.

PharmaVoice
AUGUST 8, 2024
Through a new deal, Roche has exclusive rights to Sangamo molecules designed to repress the gene that makes “tau,” a protein many scientists view as a main driver of Alzheimer’s.

Pharmacy Times
AUGUST 8, 2024
Tirzepatide demonstrates statistically significant improvements in adult patients who have heart failure with preserved ejection fraction and obesity.

Advertisement
Are you still using workarounds to manage your daily operations? To achieve peak performance, it's time to explore other options for specialty and infusion pharmacy software. Streamline pharmacy operations and improve clinical performance with automated processing, real-time data exchange, and electronic decision support. Download this helpful infographic to: Drive efficiency and patient adherence from referral receipt to delivery and ongoing care – all with our Pharmacy Cloud.

Fierce Healthcare
AUGUST 8, 2024
Though more than half of workers believe employer support for substance and alcohol issues is important, only 14% have access to such a benefit, a new survey finds. | The survey found that about a third of the workforce—or 60 million Americans—experience personal or family issues related to substance or alcohol use. This causes losses in productivity and increases in healthcare costs.

Fierce Pharma
AUGUST 8, 2024
While a glut of drugmakers have raised their 2024 financial guidance after the second quarter, those upgraded sales expectations pale in comparison to Eli Lilly’s new forecast for the year. | Lilly, which reported a 36% sales increase year-over-year to $11.3 billion in the second quarter, is raising its guidance for the full year by a staggering $3 billion.

STAT
AUGUST 8, 2024
Two of the leading companies in the AI drug development field, Recursion Pharmaceuticals and Exscientia, said Thursday that they will merge into one company. The new company will continue using the Recursion name and be led by Recursion co-founder and CEO Chris Gibson. Exscientia’s interim CEO, David Hallett, will serve as the company’s science chief.

Fierce Pharma
AUGUST 8, 2024
A year after it was hit with a rejection from the FDA, Citius Pharmaceuticals has scored an approval for Lymphir to treat cutaneous T-Cell Lymphoma.

STAT
AUGUST 8, 2024
WASHINGTON — A federal judge on Thursday tossed out a U.S. Chamber of Commerce lawsuit challenging Democrats’ drug pricing law. The decision is yet another loss for the pharmaceutical industry and its allies, which have filed lawsuits across the country arguing that the Inflation Reduction Act, which created a drug price negotiation program in Medicare , is unconstitutional.

pharmaphorum
AUGUST 8, 2024
Recursion Pharma has agreed to merge with rival AI-powered drug discovery firm Exscientia in an all-stock deal valued at $688 million

STAT
AUGUST 8, 2024
This is Part 3 of “Behind the Counter,” an in-depth video series demystifying the complex world of patents and drug pricing. Discovering a new drug is a big win for a pharmaceutical company, but selling it for lots of money with no competition? The cherry on top. This kind of market exclusivity is only possible because of patents.

pharmaphorum
AUGUST 8, 2024
Roche is said to be considering a sale of cancer data specialist Flatiron Health, just a few years after it bought the company for $1.

STAT
AUGUST 8, 2024
Hunger killed an estimated 4 million people in Ukraine between 1932 and 1933 — the result of Holodomor, a famine inflicted by the Stalin-led Soviet regime. New research shows how the harm experienced during famines can extend even to people who haven’t yet been born. The study , published Thursday in Science, strengthens the link between fetal exposure to famine and increased risk for type 2 diabetes later on in life.

Fierce Healthcare
AUGUST 8, 2024
Private equity firm Prospect Medical Holdings has entered into an agreement to sell off Crozer Health to CHA Partners. | Prospect Medical Holdings has entered into an agreement to sell off Crozer Health to CHA Partners.

STAT
AUGUST 8, 2024
Eli Lilly raised its full-year revenue guidance by $3 billion, driven by massive sales of its GLP-1-based diabetes and obesity drugs Mounjaro and Zepbound. The pharma giant on Thursday boosted its forecast for 2024 sales to a range of $45.4 billion to $46.6 billion from the previously guided range of $42.4 to $43.6 billion. It also raised its guidance for adjusted earnings per share to a range of $16.10 to $16.60 from the previously guided $13.50 to $14.00.

Fierce Pharma
AUGUST 8, 2024
While Apellis Pharmaceuticals has spent much of the year working around setbacks that derailed the ongoing launch of its geographic atrophy (GA) drug Syfovre, a major phase 3 triumph could open up | Empaveli showed strong efficacy in two rare kidney diseases. The result “exceeded our already high expectations," an Apellis exec said, and it led analysts at Evercore ISI to assign the drug a majority future market share against Novartis’ rival.

STAT
AUGUST 8, 2024
Digital Therapeutics developer Big Health this week received Food and Drug Administration clearance for SleepioRx, a prescription treatment for insomnia. Big Health already sells a wellness version of the app, called Sleepio, to employers and health plans that make the product available to their members. The app delivers a specialized type of cognitive behavioral therapy for insomnia and the company boasts that it is backed by dozens of studies.

Fierce Healthcare
AUGUST 8, 2024
The Centers for Medicare and Medicaid Services released a final procedural notice on Wednesday to expedite Medicare coverage of novel medical devices and get the devices to Medicare benef | CMS released a final procedural notice on its TCET rule, which would expedite coverage for FDA Breakthrough medical devices. A bill is also making its way through Congress that would replace the rule.

Fierce Pharma
AUGUST 8, 2024
After taking a market share lead in the crowded Humira biosimilar space and with other key launches in the works, Novartis spinout Sandoz is raising its full-year sales guidance. | The company touts leading biosimilar market share for its version of AbbVie's Humira.
Let's personalize your content