Intensive lowering of blood pressure tied to lower dementia risk
STAT
APRIL 21, 2025
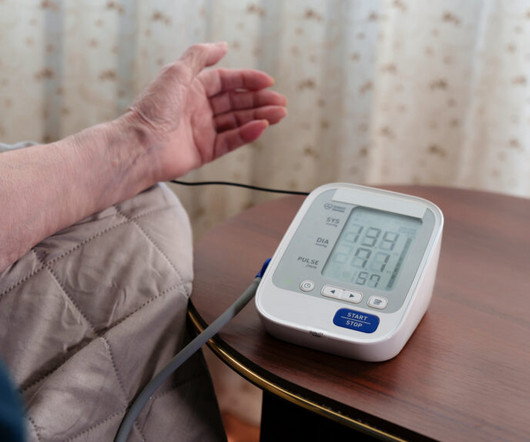
High blood pressure earned its reputation as the silent killer by causing heart attacks, heart failure, and strokes. It’s also been a suspect in dementia. Some studies have hinted at a correlation between lower blood pressure and fewer dementia cases, but they were too small and too short to lend statistical significance to the link. It’s also been noted that people with untreated high blood pressure carry a 42% higher risk of developing dementia.






































Let's personalize your content