Deucravacitinib Shows Joint, Skin Symptom Improvements in Psoriatic Arthritis
Drug Topics
JUNE 11, 2025
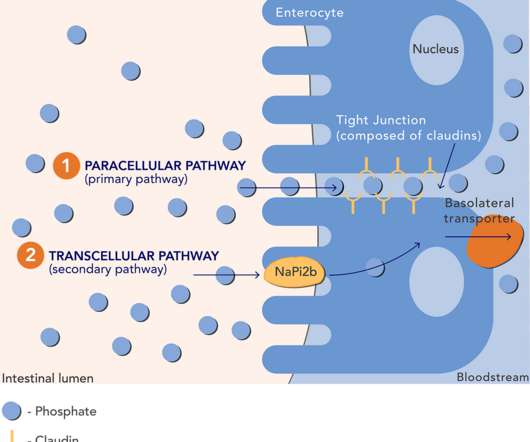
POETYK PsA-1 is a randomized, double-blind, placebo-controlled phase 3 trial evaluating the efficacy and safety of deucravacitinib compared to placebo in adult patients with PsA who are naïve to biologic disease-modifying anti-rheumatic medications. of patients who received placebo. References 1.


















Let's personalize your content