New Trends & Requirements from Digitalization, Annex 1, Continuous Manufacturing & High Potent Manufacturing
ISPE
MARCH 16, 2023
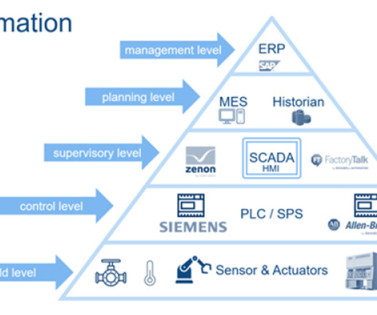
The document provides specific guidance on the sampling and testing procedures for environmental monitoring, as well as the limits for particular and microbial contamination. Automated systems offer a range of options for pharmaceutical companies. Environmental monitoring is another important aspect of Annex 1.






















Let's personalize your content