Important factors to consider when working with CRFs
pharmaphorum
AUGUST 10, 2020
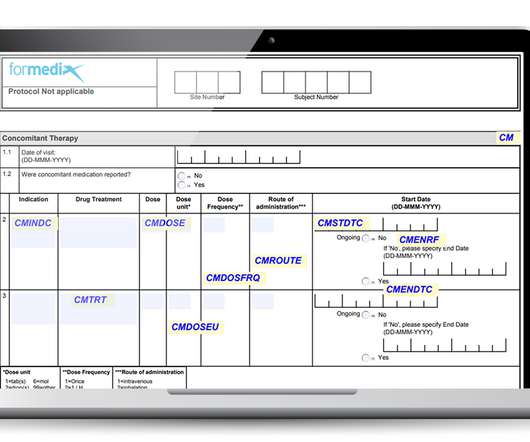
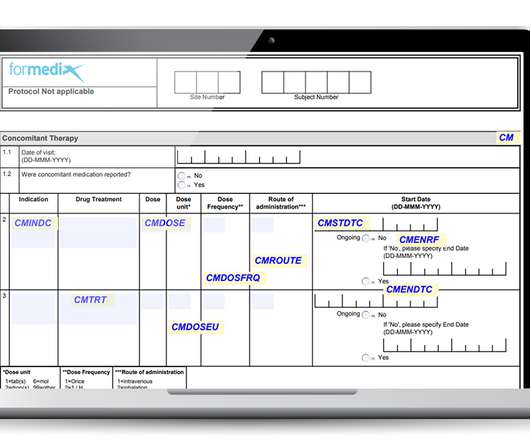
They must also comply with regulatory requirements, such as those defined by the FDA. Well-designed forms must: Gather data that’s complete, accurate, and of high quality. Well-designed forms must: Gather data that’s complete, accurate, and of high quality. Be unambiguous and allow for accurate data entry.










Let's personalize your content