Considerations for safety data migration methods
European Pharmaceutical Review
SEPTEMBER 26, 2023
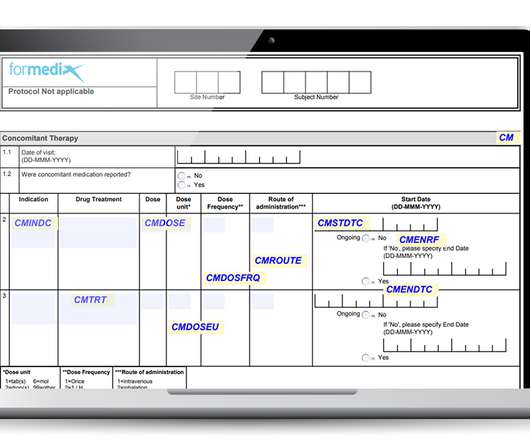
Any discrepancies should be documented using a Known Difference document and the solutions or acceptance of the discrepancy are then agreed upon. Once all cases have been entered and reviewed and any discrepancies resolved, the data transfer is considered complete and a data migration summary report should be issued.











Let's personalize your content