FDA-Approved Labeling: Is Enough Enough?
The FDA Law Blog
AUGUST 21, 2023
Livornese — I saw the sign…and the answer is no—FDA-approved labeling apparently is not enough under state failure-to-warn laws, according to certain courts.

The FDA Law Blog
AUGUST 21, 2023
Livornese — I saw the sign…and the answer is no—FDA-approved labeling apparently is not enough under state failure-to-warn laws, according to certain courts.

pharmaphorum
AUGUST 27, 2020
Already under fire for what some view as a premature authorisation of convalescent plasma for COVID-19, the FDA is now being accused of a blunder that could render current supplies unusable. Now, tragically, there is zero credibility for the upcoming @US_FDA review of #SARSCoV2 vaccines in the months ahead.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
European Pharmaceutical Review
SEPTEMBER 22, 2023
Limitations of monoclonal antibody therapies Regulatory approvals from the US Food and Drug Administration (FDA) for aducanumab and lecanemab – and likely very soon for donanemab also – opened a route for different therapeutic modalities and other relevant disease targets, such as tau.
pharmaphorum
JANUARY 8, 2021
As COVID-19 vaccines are hastily deployed in the UK for priority groups, a debate rages over the government’s controversial strategy to delay time between vaccine doses. When the UK announced the approval of the Pfizer-BioNTech and Oxford/AstraZeneca COVID-19 vaccines, it marked an exciting moment for the nation.
pharmaphorum
JANUARY 8, 2021
As COVID-19 vaccines are hastily deployed in the UK for priority groups, a debate rages over the government’s controversial strategy to delay time between vaccine doses. When the UK announced the approval of the Pfizer-BioNTech and Oxford-AstraZeneca COVID-19 vaccines, it marked an exciting moment for the nation.

PharmaShots
APRIL 3, 2023
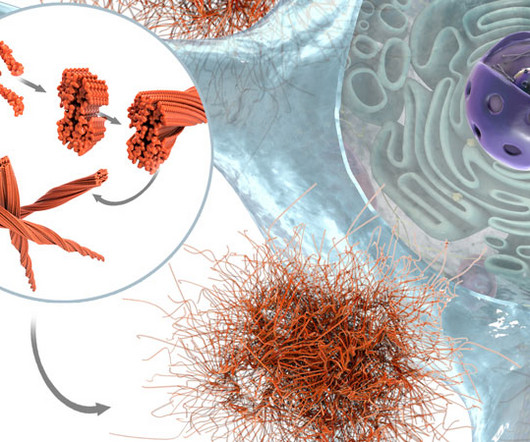
of the newly combined company The combined companies’ focus is to advance Elicio’s lymph node-targeting amphiphile technology to develop immunotherapies & also focus on ELI-002, a therapeutic cancer vaccine targeting mKRAS-driven tumors is currently being evaluated in the P-I trial (AMPLIFY-201) for PDAC and CRC. ORR (41.8%

The Checkup by Singlecare
MARCH 28, 2024
Food and Drug Administration (FDA) to treat many bacterial infections. Amoxicillin is FDA-approved for treating nose, throat, ear, lung, skin, and urinary tract infections. It may also be prescribed off-label to treat other bacterial infections, including certain STDs. Amoxicillin is an antibiotic approved by the U.S.
Let's personalize your content