Pharmaceutical Procurement Strategies Overview
Viseven
SEPTEMBER 14, 2023
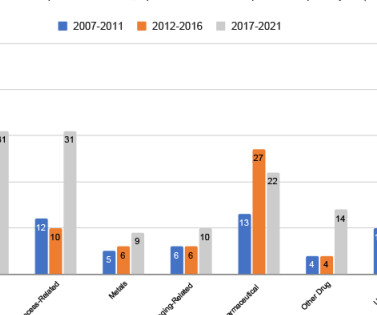
It also bears extra replacement expenses when drugs are discarded due to improper packaging. Hidden expenses can pile up, spiraling out of control due to many reasons related to supplier performance: the lack of appropriate documentation, storage, wrong dosage forms, and the list goes on.











Let's personalize your content