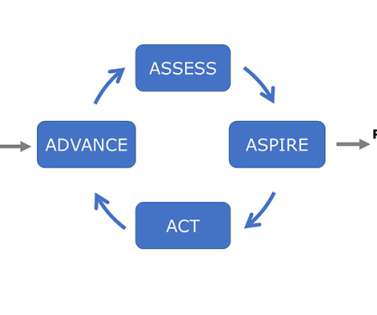
AI in Medical Affairs: Revolutionizing Life Sciences with AI-Powered Tools
Viseven
JUNE 18, 2025
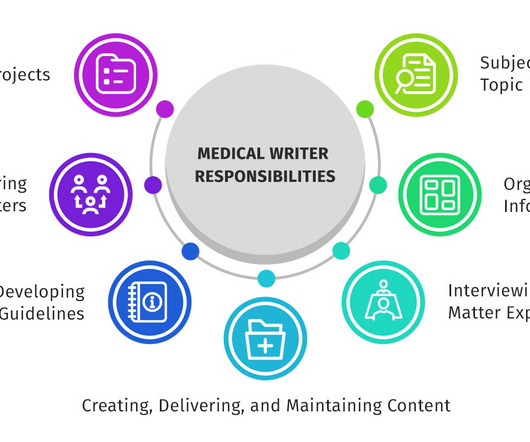
Scientific communication : Drafting slide decks, scientific responses, medical information letters, and training content using generative models, ensuring scientific accuracy and compliance in messaging and content.












































Let's personalize your content