Pharma manufacturing can digitalise its way round the next crisis
pharmaphorum
DECEMBER 29, 2022
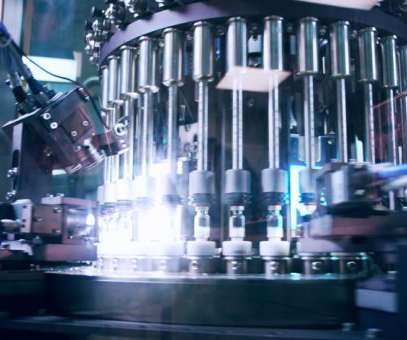
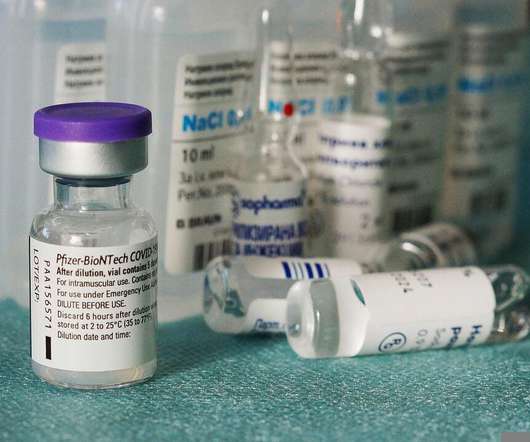
These therapeutic innovations increase demand for greater flexibility, agility, and transparency – greater than is currently available in most manufacturing environments. Process analytical technology to boost efficiency. Its engineers employed these insights to design mechanical improvements.
















































Let's personalize your content