FDA Unveils Its Own Medical Queries—A Standardized Approach for Grouping MedDRA Preferred Terms that Will Impact NDA/BLA Safety Analyses and Drug Labeling
The FDA Law Blog
SEPTEMBER 23, 2022
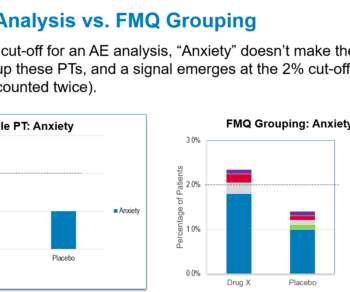
For a number of years, FDA has been including groups of related preferred terms in tables in the Adverse Reactions Section of drug labeling (Section 6), generally describing such groupings with the use of footnotes. Such groupings have generally seemed to appear in labeling on an ad hoc basis, without standardization.













Let's personalize your content