Pharma Marketing AI: Doing More With More
Pharma Marketing Network
JULY 18, 2025
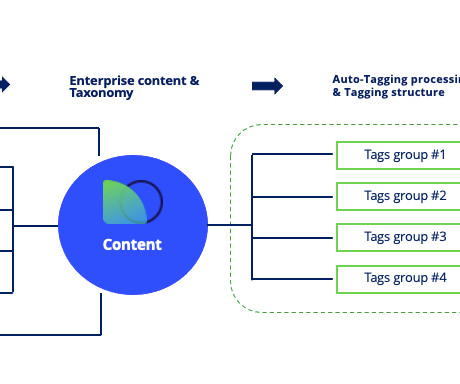
Fortunately, AI is stepping in to support medical-legal review (MLR) workflows and ensure regulatory alignment. Smarter MLR Workflows Review cycles that once took weeks can now be accelerated by AI-based content tagging and document comparison tools. Yes, when deployed with HIPAA-compliant platforms and ethical frameworks.
















Let's personalize your content