FDA Approves Updated Label for Altuviiio for Effective Bleed Protection in Children Younger than 12 Years
Drug Topics
MAY 13, 2024
Altuviiio was first granted approval by the FDA in February 2023.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Drug Topics
MAY 13, 2024
Altuviiio was first granted approval by the FDA in February 2023.

Drug Topics
APRIL 5, 2023
Off-label drug combinations are increasingly being used in cancer treatments, but there is the potential for overlapping or additive toxicities, explained a speaker at the 2023 HOPA Annual Conference.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
pharmaphorum
AUGUST 2, 2023
Samsung seeks 'interchangeable' label for Humira biosimilar Phil.Taylor Wed, 02/08/2023 - 10:37 Bookmark this

pharmaphorum
JUNE 28, 2023
AbbVie, Genmab eye label expansion for Epkinly Phil.Taylor Wed, 28/06/2023 - 13:04 Bookmark this

pharmaphorum
AUGUST 25, 2023
CVS Health launches private label biosimilars unit Phil.Taylor Fri, 25/08/2023 - 08:07 Bookmark this

pharmaphorum
NOVEMBER 17, 2023
AZ first to AKT finish line, but FDA clears narrow label Phil.Taylor Fri, 17/11/2023 - 09:46 Bookmark this

Drug Store News
JULY 26, 2023
Dollar General is debuting more than 100 new items in its Clover Valley private label line in 2023.

pharmaphorum
JUNE 6, 2023
ASCO: Enhertu data sets up tumour-agnostic label bid Phil.Taylor Tue, 06/06/2023 - 08:26 Bookmark this

Drug Store News
JULY 6, 2023
Several beauty trends will be revealed at PLMA’s 2023 annual Private Label Trade Show, Nov. 12-14 in Chicago.

Fierce Pharma
MAY 15, 2023
UPDATED: After Supreme Court rejection, Teva mulls options in GSK 'skinny label' feud fkansteiner Mon, 05/15/2023 - 10:59

Fierce Pharma
JANUARY 4, 2023
Particulates, label issues prompt separate recalls for Pfizer's Hospira and Accord. Wed, 01/04/2023 - 11:35.

Drug Store News
MARCH 23, 2023
The award recognizes the men and women who have significantly contributed to the growth and development of the global private brand industry.
Pharmaceutical Technology
MARCH 22, 2023
In the US Drug Quality and Security Act (DSCSA), the FDA mandates that manufacturers and trading partners should have full interoperable electronic track and trace systems in place by November 2023. RFID is an important facet of smart labelling and its evolution, but not the only one.

Fierce Pharma
MARCH 20, 2023
Novo Nordisk, Eli Lilly replenish Type 2 diabetes stars Ozempic, Mounjaro after off-label weight-loss boom fkansteiner Mon, 03/20/2023 - 10:09

NF2 BioSolutions
JANUARY 4, 2023
Print our cake labels, bunting and flyers to make your event a great success. The post 2023 Fundraising Challenges appeared first on NF2 BioSolutions. You can set up a personal fundraiser on Peoples fundraising or simply choose the existing NBS UK fundraiser. Buy, bake or ask for donations of cakes.
Pharmacy Times
MAY 13, 2024
Antihemophilic factor (recombinant) Fc-VWF-XTEN fusion protein-ehtl was initally approved in February 2023 for adults and children with hemophilia A for prophylaxis and on-demand treatment to control bleeding.

OctariusRx
AUGUST 28, 2023
The 2023 version of the OctariusRx medication safety quiz is about to be released. What does the Medication Safety Quiz 2023 cover and how do you get one? The quiz focuses on some of the most commonly encountered situations, including allergies, labeling medications and controlled substances. How are you assessing your staff?
The Checkup by Singlecare
DECEMBER 6, 2023
In 2023, the top medications with SingleCare customers included vitamin D supplements, inhalers, and anti-inflammatory drugs. It’s also often prescribed off-label—that is, for a use other than what it’s been approved for—as a treatment for fibromyalgia. Read on to see if any of your prescriptions made the list. Levothyroxine 1.

Fierce Pharma
MARCH 31, 2023
In Teva vs. GSK skinny label feud, Biden admin urges Supreme Court to weigh in fkansteiner Fri, 03/31/2023 - 09:11

The FDA Law Blog
DECEMBER 8, 2022
Food and Drug Administration (FDA) issued two guidance documents, one draft and one final, on food allergen labeling requirements. 1, 2023; The applicability of food allergen labeling requirements to specific products (e.g., 1, 2023; The applicability of food allergen labeling requirements to specific products (e.g.,

European Pharmaceutical Review
OCTOBER 24, 2023
Europe’s pharmaceutical packaging and labelling market is projected to reach $35.78 billion in 2022, the pharmaceutical packaging and labelling market in Europe is expected to expand at a compound annual growth rate (CAGR) of 4.78 percent between the forecast period: 2023 and 2028. percent between the forecast period 2023-2028.

European Pharmaceutical Review
AUGUST 30, 2023
The Windsor Framework, announced on 9 June 2023 by the Medicines and Healthcare products Regulatory Agency (MHRA), provides a long-term solution for the supply of medicines into Northern Ireland. The agreement stipulates that certain conditions must be met in the labelling and packaging of these medicinal products.

Pharmaceutical Technology
DECEMBER 4, 2022
Humira is indicated for 10 adult and pediatric conditions, including Crohn’s disease, rheumatoid arthritis, and ulcerative colitis, as per its label. Indeed, Amgen’s biosimilar was approved in September 2016, but the company was not able to launch it until January 2023 in the US. Temporary first-to-market advantage.
Pharmaceutical Technology
APRIL 17, 2023
The US Food and Drug Administration (FDA) has approved an update to the indications and usage section of Horizon Therapeutics ’ Tepezza (teprotumumab-trbw) label to specify its use to treat thyroid eye disease (TED) patients regardless of disease activity or duration. The FDA’s approval was granted in January 2020.

Fierce Pharma
FEBRUARY 2, 2023
Merck's recent Keytruda label expansion in NSCLC is more of a 'long-term' opportunity, CEO says kdunleavy Thu, 02/02/2023 - 10:43

Hospital Pharmacy Europe
MARCH 26, 2024
It is likely to be safe for penicillin allergy labels (PALs) to be removed for most patients, a study from the University of Birmingham has found. Some 97% of participants labelled with PALs were shown to have no allergy. However, a large body of evidence suggests that 90-95% of PALs are inaccurate.

The Checkup by Singlecare
DECEMBER 6, 2023
In 2023, medications like Ozempic , Mounjaro , and Wegovy soared in popularity—so much so that many pharmacies faced a shortage. Read on to see what readers cared about the most in 2023. Whether you’re taking Ozempic for diabetes or off-label for weight loss, you might be curious if it’s safe to mix it with alcohol.

Fierce Pharma
FEBRUARY 6, 2024
billion in sales, bringing its 2023 haul to $8.9 On a day when Vertex revealed trial data that indicate | On a day when Vertex revealed trial data that indicate its next-generation cystic fibrosis (CF) treatment will soon be ready to grab the baton, the Boston company also presented figures that show Trikafta remains formidable.
European Pharmaceutical Review
JULY 4, 2023
The International Organization for Standardization (ISO) has published its new standard Sterilization of health care products — Microbiological methods — Part 3 Bacterial endotoxin testing ( ISO 11737-3:2023). This includes products that must be non-pyrogenic based on either intended use or non-pyrogenic label claim, or both.

pharmaphorum
MAY 10, 2021
billion doses through 2023. Happy to announce that @EU_Commission has just approved a contract for guaranteed 900 million doses (+900 million options) with @BioNTech_Group @Pfizer for 2021-2023. The meeting did however recommend a change to the label for Comirnaty to include a risk of facial swelling with the jab.

The FDA Law Blog
JANUARY 2, 2023
Labeling in the drug and biologics approval process. FDA Boot Camp is taking place virtually from March 22-23, 2023, visit [link] to learn more! Clinical trials for drugs and biologics. cGMPs and other manufacturing concerns relative to products liability. Proactive adverse events monitoring and signal detection.

STAT
JANUARY 25, 2024
One person also experienced hypoglycemia in 2023 after injecting a compounded version of Ozempic, said the organization, which represents 55 regional poison centers across the country and works with the U.S. Ozempic and similar diabetes medicines have been increasingly used off label for weight loss.

Pharmaceutical Technology
APRIL 17, 2023
On 14 April 2023, experts from the US Food and Drug Administration’s (FDA) Advisory Committee (AdCom) voted largely in favour of the potential approval of Otsuka’ s and Lundbeck Pharmaceuticals’ Rexulti for the treatment of agitation associated with Alzheimer’s dementia (AAD). Rexulti is an atypical antipsychotic.
BioPharm
OCTOBER 3, 2023
The new customized system is designed for fully automated and integrated labeling, orientation, and palletization, and it is expected to streamline the current manual processes, according to the press release.
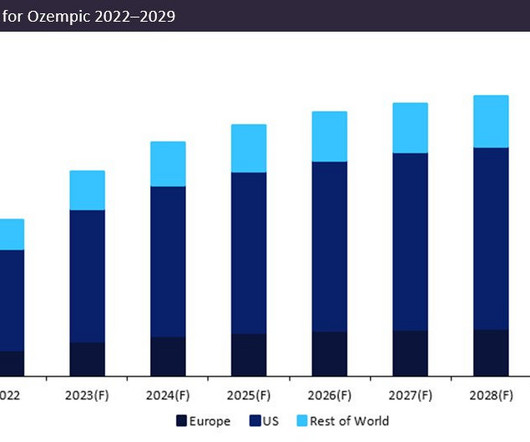
Pharmaceutical Technology
APRIL 28, 2023
Novo Nordisk’s leading drug Ozempic (semaglutide) is forecast to demonstrate a sales growth of 23% in 2023. Ozempic’s forecast 2023 sales of $12.5bn consolidate its position as the dominant market leader, with projected sales in 2023 54% greater than closest competitor Trulicity (dulaglutide) by Eli Lilly, which anticipates sales of $8bn.
Hospital Pharmacy Europe
JANUARY 4, 2024
The alert supersedes previous guidance issued in July 2023 instructing prescribers not to initiate any new patients on GLP-1 RAs for the duration of the shortage, which was then predicted to continue until at least mid-2024. And it warned prescribers not to prescribe GLP-1 RAs licensed for type 2 diabetes for off-label indications.

Pharmaceutical Technology
DECEMBER 11, 2023
Novartis is seeking approval for Kisqali as a treatment of early breast cancer, with an FDA application planned by the end of 2023.

The Checkup by Singlecare
DECEMBER 1, 2023
Department of Agriculture (USDA) requires it to be labeled “farmed” or” wild-caught.” Check the nutrition facts label to find the amount of vitamin D and other nutrients contained in individual products. As of January 2023, an increase of up to 560 IU of vitamin D per 100 g of cereal is allowed. mcg or 100–144 IU per cup.

European Pharmaceutical Review
OCTOBER 19, 2023
According to the European Medicines Agency (EMA), pre-filled pens falsely labelled as the type 2 diabetes medicine Ozempic ( semaglutide , 1mg, solution for injection) have been identified at wholesalers in the EU and the UK. The pens, with labels in German, originated from wholesalers in Austria and Germany.
European Pharmaceutical Review
MARCH 8, 2023
A placebo-controlled, Phase IIa, proof-of-concept, social anxiety clinical trial is expected to begin in the first half of 2023. The results are anticipated by the end of 2023. An open-label, Phase IIa, proof-of-concept clinical trial in women with PPD is expected to initiate in the second half of 2023.
European Pharmaceutical Review
OCTOBER 18, 2023
Further to the three Phase III trials for XPHOZAH, Ardelyx also declared that it has completed two open-label clinical trials (OPTIMIZE and NORMALIZE) to evaluate different options for integrating the treatment into clinical practice. XPHOZAH is expected to be available to eligible patients in the US in November 2023.

PharmaShots
MAY 25, 2023
AbbVie’s Rinvoq (upadacitinib) Receives EC’s Approval for the Treatment of Moderately to Severely Active Crohn's Disease Rinvoq Active ingredient: upadacitinib Approved: April 17, 2023 Company: AbbVie Disease: Active Crohn's Disease The approval was based on 3 P-III trials incl. (U-EXCEED and (15 & 30mg) @52wks.

European Pharmaceutical Review
APRIL 19, 2023
percent from 2023, according to the data. This is driving the adoption of item-level smart labelling , the report noted. A market report has identified that global demand for electronic smart packaging will reach $2.6 billion in 2033. This represents a compound annual growth rate (CAGR) of 15.4

PharmaShots
APRIL 5, 2023
The results are expected at the end of 2023 Additionally, MiGenTra and Minapharm will be sharing responsibilities to file, launch and commercialize Adessia in Africa and the Middle East. The company's main goal is to advance the most diverse pipelines in neuroscience, which will revolutionize patient care in a no.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content