Life-Cycle Approach to Cleaning Topical Drug Products
ISPE
JANUARY 11, 2023
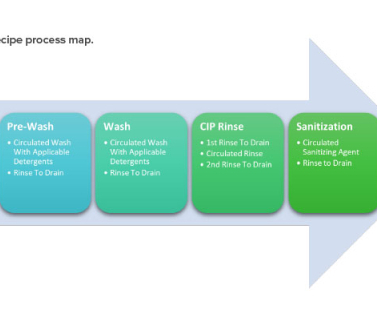
Life-Cycle Approach to Cleaning Topical Drug Products Trudy Patterson Wed, 01/11/2023 - 10:17 Technical January / February 2023 Life-Cycle Approach to Cleaning Topical Drug Products Dijana Hadziselimovic Kamini I. The cleaning validation life-cycle approach consists of three stages: design, qualification, and continued verification.








Let's personalize your content