Life-Cycle Approach to Cleaning Topical Drug Products
ISPE
JANUARY 11, 2023
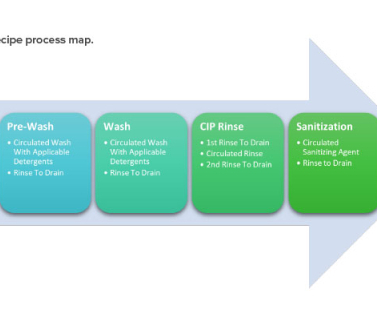
The cleaning validation life-cycle approach consists of three stages: design, qualification, and continued verification. Often referred to as the validation stage, stage two: qualification confirms that the cleaning procedure under normal conditions meets preestablished acceptance criteria. 11 (2017): 36–46. 10 Rivera, E.,









Let's personalize your content