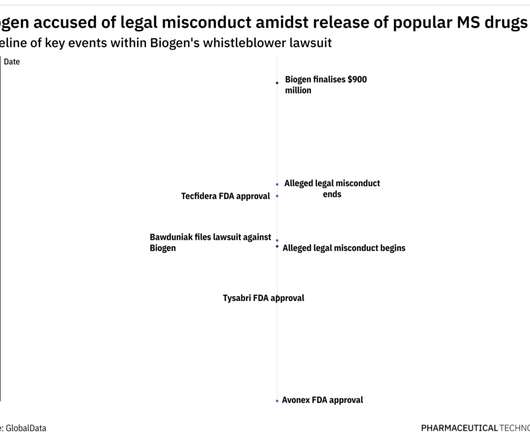
PDA revises report on glass container manufacturing
European Pharmaceutical Review
JULY 5, 2023
Having been originally published in 2007, then revised in 2013, this latest 2023 edition addresses evolving standards and container types. According the PDA, the standardised quality criteria in TR 43 are intended as a guidance overview for container manufacturers and for incoming container acceptance inspection at pharmaceutical companies.














Let's personalize your content