Time to change the channel? The future for customer engagement models
pharmaphorum
OCTOBER 20, 2020
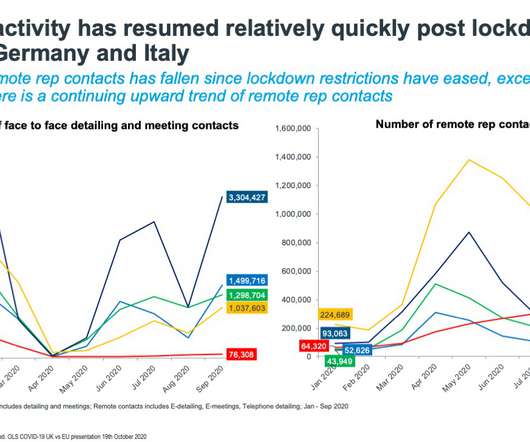
The pandemic has triggered a change in how pharmaceutical companies are engaging with healthcare professionals. When IQVIA polled non COVID-19 treating Italian HCPs in March when the crisis was at its worst, it asked HCPs if they still welcomed pharmaceutical company engagement during the crisis. The answer was yes.

















Let's personalize your content