Methodology to Define a Pharma 4.0™ Roadmap
ISPE
MAY 11, 2023
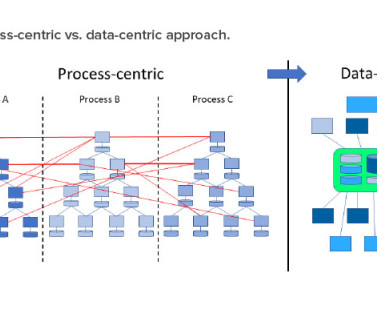
Published June 2008. ISPE GAMP® Guide: Records and Data Integrity. Based on robust data flows, aspects and required controls can be assessed in detail—for example, manual data entry, interfaces between systems, media change, data conversion, data migration, and data archiving. to Industry 4.0







Let's personalize your content