Remote Acceptance Testing of Automation Projects
ISPE
OCTOBER 28, 2022
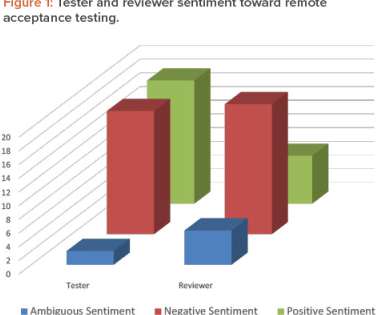
The writing of requirements, design documentation, and test scripts, and the configuration of hardware and software, can be conducted from home. Audio-visual communication. Text communication. Technology and communications requirements. Technology and communication requirements. Table 3: Codes. Advantages.



















Let's personalize your content