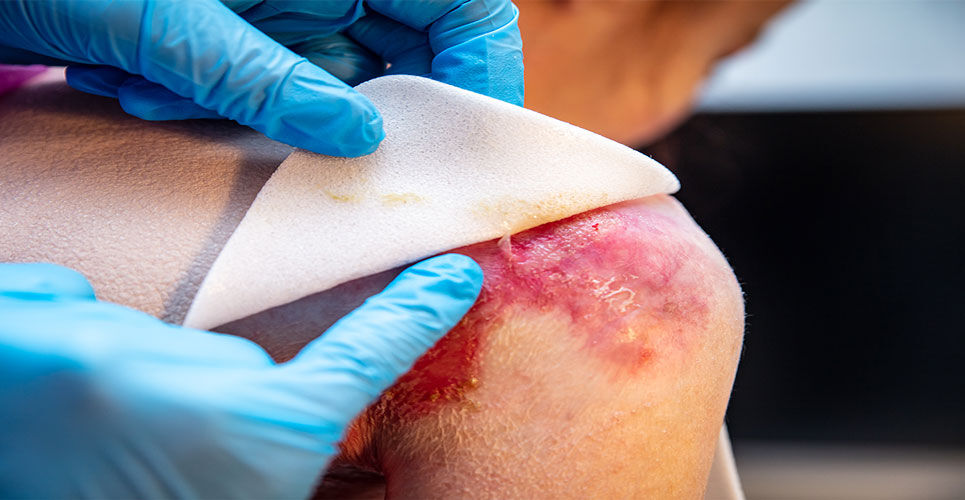
A birch bark extract has been recommended in the UK by the National Institute for Health and Care Excellence (NICE) for treating skin wounds associated with dystrophic and junctional epidermolysis bullosa.
The final draft guidance recommends the use of Filsuvez Gel, used within its marketing authorisation, as an option for treating partial thickness wounds associated with dystrophic and junctional epidermolysis bullosa in adults and children aged six months and over.
The extract represents the first treatment recommended by NICE for epidermolysis bullosa.
Commenting on the final draft guidance, Helen Knight, director of health technology assessment at NICE, said: ‘[This] is yet another example of how NICE is able to drive innovation into the hands of health and care professionals and get the best care to people fast, striking a balance between effectiveness and the best use of public funding.
‘Birch bark extract, by reducing the time taken for wounds to heal and therefore the time needed to clean and redress them and the pain associated with that, has the potential to improve the quality of life for people with EB and their carers and help free up time for other activities.‘
The final NICE guidance is expected to be published on 20 September 2023.
Epidermolysis bullosa is a rare genetic, blistering skin disorder that is usually present from birth. Affected patients experience mechanical fragility of epithelial surfaces including the skin and, as a result, extensive and recurrent blistering.
Birch bark extract approval
The NICE recommendation of birch bark extract was based on effectiveness data from the phase 3 randomised double-blind EASE trial.
A total of 223 enrolled patients had either dystrophic, junctional or Kindler epidermolysis bullosa and a target partial-thickness wound lasting ≥ 21 days and < 9 months,that was 10-50 cm2. They were randomised 1:1 to birch bark gel (Oleogel-S10) or a control gel, both of which used standard-of-care dressings. A total of 109 patients were in the birch bark group and 114 in the control group. The gel was applied to all wounds at least every four days.
The primary endpoint was the proportion of patients with first complete closure of the target wound within 45 days.
After 45 days, 41.3% of patients treated with the birch bark extract had complete target wound closure, compared with 28.9% in the control gel arm (p = 0.013).
Adverse events occurred with similar frequency for both the birch bark extract and the control gel (81.7% vs 80.7%), although these were mainly of mild-to-moderate intensity.
Recently, the FDA approval of the first novel topical gene therapy provided hope for dystrophic epidermolysis bullosa sufferers.