Artificial Intelligence is Changing the Face of Pharmacovigilance
Pharmaceutical companies are looking into AI as the new method to not only reduce research and development costs, but also prevent costly errors.
Background
The pharmaceutical landscape is constantly evolving with an increased volume and complexity of new drugs and therapeutics becoming available on the market on an annual basis. With innovation and the advancement of the industry, concerns regarding drug safety arise with adverse events (AEs), commonly referred to as pharmacovigilance (PV).
Between 2009 and 2019, the number of AEs recorded by the FDA Adverse Event Reporting System (FAERS) increased by more than 300%.1 In 2021, more than 2.2 million AE reports were submitted to FAERS (Figure 1).2
Figure 1. FDA Adverse Events Reporting
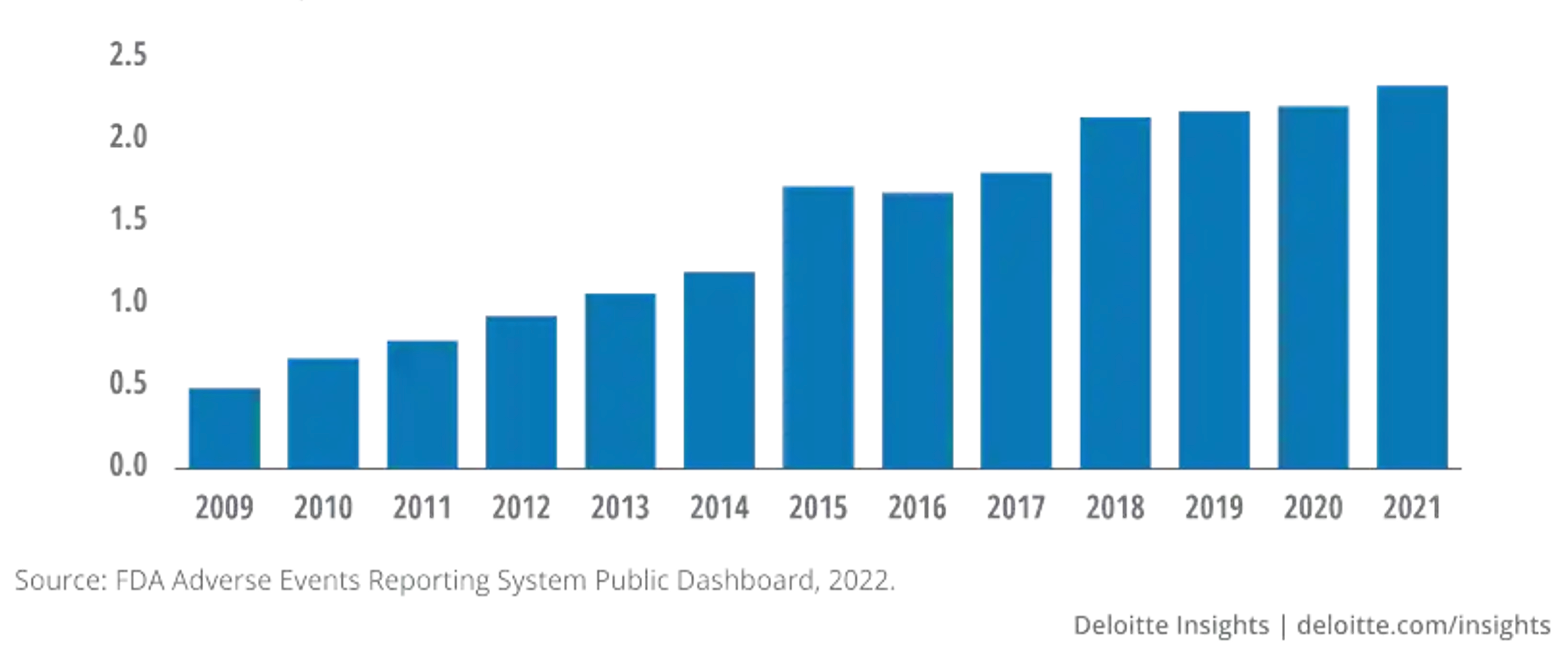
With this rise in volume of reported AEs, there is an increased burden placed on PV and drug safety professionals to accurately and efficiently evaluate data.3 Therefore, it has become paramount to implement new technologies to reduce the workload on these professionals while also ensuring precise and systematic evaluation of the data present at hand.3
Fortunately, while there have been great advancements in the pharmaceutical industry, the advancements in the tech industry have also been paralleled. To keep up with this high volume of data collection and interpretation, the use of artificial intelligence (AI) is being implemented in the pharmaceutical industry.
AI is prevalent in many areas of pharmacy and has assisted in navigating complex challenges, including drug shortages, drug recalls, and even the opioid epidemic.4 The challenge now involves successfully applying AI technologies and strategies to the PV industry to ensure sustainable outcomes for drug safety.
Introduction to PV
PV is defined as the science relating to the detection, assessment, understanding, and prevention of adverse drug events.5 This branch of the drug development and safety process focuses on observing trends in AEs caused by a specific drug.
The main goal of PV is to identify these trends of adverse drug reactions (ADRs) and reduce their occurrence and threat to public safety in a prompt manner throughout the entire life cycle of the drug.6 There are many components involved in PV, including clinical trial data review, evaluation of literature, risk management, and individual case reporting.6
The basis of pharmacovigilance relies on the reporting of ADRs by health care professionals, patients, and caregivers from sources such as clinical trials, real world evidence, and even patient support groups. Nearly 7% of all hospital admissions is due to ADRs in the United States.7 Drug safety is a major concern for pharmaceutical companies, regulatory authorities, public officials, and patients during the drug development process and post-marketing phase.
The PV function was first introduced about 174 years ago in 1848 when a young girl consumed chloroform anesthetic before the removal of her infected toenail. While the cause of the girl’s death was investigated, it was difficult to identify and reach a conclusion.
Along with other clinicians’ concerns of deaths caused by the anesthesia, these results were published in The Lancet. Shortly after in 1906, the US Federal Food and Drug Act was created and a system was established in which the safety of drugs needed to be shown before their premarket approval.8
About 50 years later, the department of PV underwent huge change in response to a global tragedy regarding the medication thalidomide.5 Thalidomide was initially on the market to cure sleeplessness and morning sickness in pregnant women; however, it ended up causing unforeseen deformities in babies and immune response suppression.
This event was the turning point in the monitoring of drug reactions and altered the system for PV. Prior to this, ADRs were reported spontaneously. Post-thalidomide tragedy, ADRs became systematic, organized, and regulated.5
Fast forward to today, there are many revolutionary technological changes being made in the drug development industry, but advancements made in the pharmacovigilance functional area have been modest.6 The US PV market worth $6.97 billion in 2022, and it is expected to reach an annual growth rate of 10.5% from 2022 to 2030.7
Individual Case Safety Reports (ICSRs) and Demands on PV
An ICSR is a legal requirement for pharmaceutical companies imposed by the FDA. ICSRs are shared among key stakeholders, including pharmaceutical companies and regulatory bodies to ensure the safety of the public. The ICSR process begins with a PV professional required to assess the validity of the case. In order for an ICSR to be valid, there are 4 minimum criteria that must be met:9
- Identifiable reporter
- Identifiable individual patient
- Suspected medicinal product
- Suspected adverse reaction
The PV professional gathers these data and develops an ICSR, which is written in a specific format consistent with the FDA’s regulations. This involves a systematic process of collecting AEs, performing appropriate research to fully comprehend the case, entering the AEs into the database, coding the AEs with standard medical terms, and classifying AEs into categories such as expected versus not expected, serious versus nonserious, and related versus non-related.6
Formulating and processing an ICSR to ensure adherence to regulatory requirements is a large contributor to the inefficiencies surrounding pharmacovigilance. With the large surge of AEs consistently reported annually, there is a concern that the development of ICSRs will no longer be sustainable measures to conduct PV analyses.
Introduction to AI
AI is a branch of computer science that enables computers and other devices to implement tasks that would otherwise require high level of human intelligence. The largest limitation that human beings have in interpreting and analyzing large amounts of data is time constraints.10
For the human mind, the process of learning involves integration of knowledge and experience acquired over a prolonged period of time. This is where AI can play a significant role because capturing vast amounts of data and reinventing the data to gain new experiences is the backbone of AI.
Therefore, computer software designed through the application of sophisticated algorithms can process an exorbitant amount of data in a significantly shorter amount of time than humans can ever acquire in their lifetimes.10 The term AI is complex and ambiguous. Although not well-defined, there are many branches and components that are a part of the larger umbrella term, which will be discussed shortly.
Specific Applications of AI in PV
Regulatory
There are many areas in which AI has started to become utilized but has not been mature enough for widespread implementation. The COVID-19 pandemic demonstrated the importance of an agile, rapid approach as new medications were fast-tracked through the FDA’s new regulatory pathways.
Even pre-pandemic, the FDA released a 5-year plan for integrating AI into the co-existing PV framework. Currently, the FDA is exploring ICSRs via their submission to FAERS to improve efficiency and scientific value of the analyses of ICSRs. Every year, the FDA receives more than 2 million reports from the industry in addition to the several hundred thousand submitted by the public.11
Clinical
It takes about 10-12 years for a new drug to be put into the market and of those, about 5 to 7 years are spent in the clinical trial phase.12 During clinical trials, patient recruitment and site selection can be time consuming, and often leads to trial failure if not done in a timely manner.
Pharmaceutical companies are looking into AI as the new method to not only reduce research and development costs, but also prevent costly errors.13 Through a data-driven approach, AI can examine large amounts of data to identify patient subgroups who may benefit from a specific clinical trial.13
Optimizing medical treatment via utilization mobile apps for measurements, AI can assist in personalizing data and ultimately impact future research and development.13
Forms of AI
Machine Learning
Machine learning is a type of AI in which computers learn and adapt over time without following explicit instructions.3 Machine learning allows software to automatically learn and improve from previous data to enhance its behavior and create new predictions when new data are available.14
This can be comparable to how the human brain is wired based on certain experiences and reviewed when presented with a new experience. Humans then subconsciously make their day-to-day decisions based on all their past experiences.15
In drug safety and PV, machine learning has many applications. Below are some examples:
- Determining which patients will benefit more from a specific treatment.
- Extracting notable data from ICSRs.
- Predicting which patients are likely to fail at screening phases of a clinical trial.
- Predicting which patients have a greater probability of discontinuing from a clinical trial.
- Predicting which patients will experience an adverse event during a clinical trial.15
When it comes to PV analytics, there are a variety of machine learning models that can be utilized: supervised learning, unsupervised learning, semi-supervised learning, reinforcement learning, natural language processing, and deep learning.
Supervised Learning
In supervised learning, labels are utilized in the algorithm to appropriately predict which label corresponds to an individual component. In this form of AI, the input of the system is tagged, or labeled, with corresponding output tags.16 For example, when predicting which individuals will experience an AE, a series of variables are associated with patients who have experienced AEs and identical sets of variables are used for patients who do not experience an AE.15
Unsupervised Learning
Unsupervised learning uses machine learning algorithms to analyze unlabeled datasets.17 Although the model is using unlabeled data, it looks for recurring patterns that exist within the input data.16
Labels are defined as classification names or group names we seek to predict. For example, when describing how serious an AE is, labels can be “serious” or “nonserious.” Some techniques that can be used are listed below:
- Cluster analysis. This is a type of data mining technique that arranges unlabeled data based on their similarities or differences.17
- Outlier detection. This is a type of data mining technique that identifies anomalies, events, or observations that differ significantly from most of the data.18
Semi-supervised Learning
Semi-supervised learning is the combination of unsupervised and supervised learning. Here, labeled data are combined with unlabeled data for the sole purpose of learning to predict models better. When there are incomplete data, predictive models can be developed with semi-supervised learning algorithms.15
Reinforcement Learning
Reinforcement learning is when prior experiences are used to achieve adequate decisions. The algorithm is ultimately improved through prior experiences with the help of a constant feedback loop.16 Focusing on specific patient traits and appearances can prevent AEs because you are tailoring toward targeted treatments.
This type of learning system focuses on policy, reward signal, value function, and an environment model.15 It can be viewed as a game-like situation in which a computer will use a trial-and-error system to come up with a solution.
The programmer provides no hints or suggestions on how to solve the game but the AI will either receive penalties or rewards for its actions. This causes the machine’s creativity to be formed through many trials. Ultimately, through multiple trials and errors, AI will start from random trials to logical tactics and skills.19
Natural Language Processing (NLP)
NLP allows computers to comprehend human language. The technique of NLP involves capturing computational linguistics to create a program that can analyze large amounts of natural language data.20
This includes recognition of speech, comprehension, translation, and interpretation and generation of language from sources such as scientific publications, health records, and even social media.20 This concept has been introduced into the tech industry since the 1960s, but has only been implemented in the PV industry within the past 10 years.20
NLP has been successful in studying suspected ADRs that demonstrated a risk of seizures with sildenafil. It has also been used to create predictive models for detection of drug-induced repolarization disorders such as torsade de pointes and sudden cardiac death.20
Deep Learning
Deep learning is a complex subset of machine learning that is meant to mimic human brain processing with the use of recurrent neural networks.10 Neural networks operate in a fashion similar to that of neurons in the brain to pass information from neuron to neuron.20
Deep learning involves the sequencing of multiple layers of machine learning algorithms that require skilled computational power.16 In deep learning, multiple data sets are processed at the same time. These data sets are then reevaluated and reprocessed in multiple rounds to achieve an output.16
Each round of data evaluation is based on the output of the previous layer, which will ultimately result in a final desired output. In drug discovery, deep learning can be used to predict structures that may have similar actions versus those that may not.20 In post market surveillance, deep learning can be used to perform comparative safety analyses and aid in clinical decision making.22
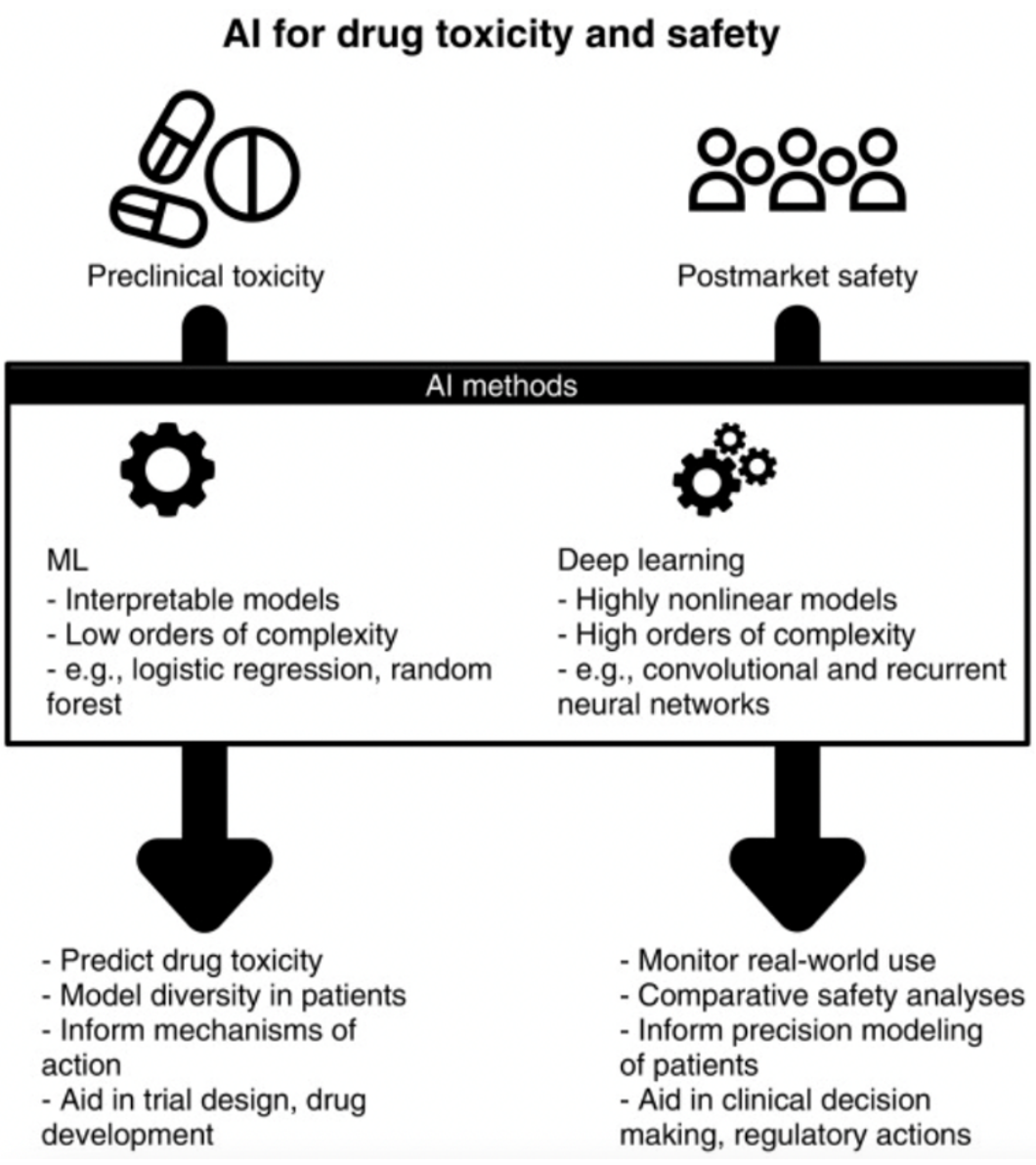
Figure 2. The use of machine learning and deep learning in preclinical toxicity and post market safety.21
Future Direction
Challenges and Limitations
It is apparent that implementing new and innovative AI technologies in the setting of PV is a necessity to keep up with demands. Although there are already many advancements made, there are still challenges and limitations that must be addressed to understand the complexities of these technologies.
Data Constraints
A major drawback when developing AI programs using sensitive protected health information is that a lot of data are required to redact information such as patient name, address, and date of birth. This makes it difficult to train models and create real-world programs that can effectively incorporate those data.9
Language Ambiguity
Interpreting PV data involves the need to process and understand complex medical information and jargon. Because of this, AI models need thorough programming to digest situational context and interpret ambiguity to ensure accurate translation of data.20
Malfunctions
Technological advancements are never foolproof and industries making use of extensive technology should also prepare for technical difficulties and malfunctions. Organizations would be required to take steps to create new support roles that have the ability to troubleshoot findings and resolve flaws in the AI algorithms.3
Training of Drug Safety Professionals
Drug safety professionals, such as pharmacists, are typically highly skilled and trained in areas such as science, pharmacy, and medicine. Most of these professionals do not have extensive training in AI technologies, nor are they required to keep up with the latest trends in AI to effectively perform their jobs. Should new and advanced AI technologies be implemented in PV, increased effort and time must be invested to train the relevant personnel.3
Impact on the Drug Safety Professional Role
For the past 20 years, there has not been much change in how drug safety professionals handle ADR reports. These reports are sent to the national competent health authority through health care professionals and drug safety professionals.
From there, the reports are transmitted, processed, and assessed for safety information to make sure they are compliant with regulations and ensure the safety of the patients. Aggregates of individual ADR reports are created and entered into the databases. Software analytics may produce further warnings that a drug manufacturer should be aware of to protect public health.3
Now with the introduction of AI, there have been many engaging conversations on how it may impact drug safety professionals and their role in PV. A main concern is whether AI technologies will eventually replace human roles and decrease the need for PV professionals.
A study conducted in 2013 at Oxford University stated up to 47% of all the jobs in the United States are at risk due to computerization.3 However, another argument states that AI will not cause humans to lose their jobs but rather create more jobs.
Researchers at Capgemini surveyed 1000 organizations that are using AI systems and noticed 80% have created new jobs and roles for the drug safety professional.3 Celgene’s Global Drug Safety and Risk Management department conducted research with members of the PV team to understand their perspectives on their roles in PV and their baseline skillset regarding the use of AI technologies.
The research methods involved a 12-part questionnaire and the results determined that additional training for core competencies and skills would be required should certain AI technologies be implemented. Participants expressed an overall positive attitude towards using AI in their daily practice to allow them to create more value-based work rather than volume-based work.3
Another recent study conducted at Celgene aimed to determine the efficacy of 10 cognitive services consisting of machine learning and natural language processing techniques to assist drug safety professionals in processing and analyzing ICSRs.9 All 10 cognitive services measured to be at least 75% accurate, which is the minimum required threshold that is needed for cognitive services to be considered adequately trained.9
Although the augmented intelligent capabilities proved to meet the minimum specifications, the intention is not to replace drug safety professionals, but rather to provide additional support during decision-making when presented with large amounts of data.9 Ultimately, the addition of AI can lead to proactive decision-making and reduced burdens on the drug safety professionals, while also ensuring compliance to regulatory requirements and optimizing patient safety.
Impact on Costs
Integrating AI in PV provides multifaceted solutions. Typically, when looking into the PV budget, case processing takes up a considerable portion. However, with automation of safety case reporting and utilizing AI learning algorithms, pharmaceutical development process costs can be decreased.22
Due to the efficiency of AI processes and PV accuracy, companies are reducing the overall cost of drug development processes. According to Deloitte, utilization of AI in PV can cut costs by 80% for the drug screening process.
AI can analyze large amounts of data while bringing a degree of automation in repetitive tasks. Hence, DS professionals can focus on more valuable objectives. Being able to tap into other outlooks aside from health care, AI will make more accurate predictions for ADRs related to a specific population.7
PV focuses on improving patient outcomes, but this comes with association of high costs because you want to have high quality ADRs. As a result, companies need to invest a lot for data analysis to occur. A recent report from Deloitte stated 90% of companies who are working with PV are figuring out solutions to cut the costs of this approach.7
Conclusion
With the current increase in AE reporting and the excessive burden placed on the PV industry, it is imperative to address the gaps in the system, and formulate solutions to combat these inefficiencies. Developing ICSRs and processing high volumes of data are a major contributor to the stagnancy in technological advancement in the PV industry.
It is evident that an optimal PV system is designed to protect subjects and patients from excessive harm, have the ability to predict trends, perform adequate risk-benefit analyses, and generate data that are useful for all stakeholders in the health care system.6 AI has the ability to transform PV through analyzing and processing large amounts of data that would normally be out of the scope of a human’s lifetime.
This is done through various methods and models that include programs such as machine learning and deep learning. Implementation of AI in PV can increase efficiency in the lives of PV professionals and allow them to perform more value-based work. It is also important to consider the logistics and limitations of carrying out such programs, including data constraints and language ambiguity.
AI in the PV industry has made numerous advancements, but this is only the beginning. There is ample room for growth and development in this industry, and it can start with the PV professional.
Experts in AI are not familiar with the nuances involved in interpreting specific clinical data, such as MRIs, CT scans, EKGs, and other laboratory parameters that might be necessary to conduct a PV analysis. Similarly, experts in PV are not well-versed in the various functions and systems involved in AI programs.
If more PV professionals are willing to branch out beyond their current scope of practice and delve into the world of AI, many milestones can be reached in the world of drug safety.
References
- Markey J and Traverso K. The untapped potential of AI & Automation Pharmacovigilance. ISPE. Pharmaceutical Engineering. August 2020. Accessed 18 August 2022.
- Taylor K, May E, Powell D, et al. Intelligent post-launch patient support. Deloitte insights. 27 July 2022. Accessed 19 August 2022. https://www2.deloitte.com/us/en/insights/industry/life-sciences/artificial-intelligence-in-healthcare-pharmacoviligance.html
- Danysz K, Cicirello S, Mingle E, et al. Artificial Intelligence and the Future of the Drug Safety Professional. Drug Saf. 2019;42(4):491-497. doi:10.1007/s40264-018-0746-z
- Zurawski D. Enter AI: How technology is changing the pharmaceutical industry for the better. Pharmacy Times. June 26, 2020. Accessed 17 August 2022. https://www.pharmacytimes.com/view/enter-ai-how-technology-is-changing-the-pharmaceutical-industry-for-the-better
- Murali K, Kaur S, Prakash A, and Medhi B. Artificial intelligence in pharmacovigilance: Practical utility. Indian J Pharmacol. 2019;51(6):373-376. doi:10.4103/ijp.IJP_814_19
- Streefland MB. Why are we still creating individual case safety reports? Clin Ther. 2018;40(12):1973-1980. doi:10.1016/j.clinthera.2018.10.012
- Avenga Team. Artificial intelligence and machine learning in pharmacovigilance: current use cases and future opportunities. 18 May 2022. Accessed 19 August 2022.
- Fornasier G, Francescon S, Leone R, et al. An historical overview over pharmacovigilance. Int J Clin Pharm. 2018; 40: 744–747. https://doi.org/10.1007/s11096-018-0657-1.
- Abatemarco D, Perera S, Bao SH, et al. Training augmented intelligent capabilities for pharmacovigilance: applying deep-learning approaches to individual case safety report processing. Pharmaceut Med. 2018;32(6):391-401. doi:10.1007/s40290-018-0251-9.
- Mintz Y and Brodie R. Introduction to artificial intelligence in medicine. Minim Invasive Ther Allied Technol. 2019;28(2):73-81. doi:10.1080/13645706.2019.1575882
- Ball R and Dal Pan G. “Artificial intelligence” for pharmacovigilance: ready for prime time?. Drug Saf. 2022;45: 429–438. https://doi.org/10.1007/s40264-022-01157-4.
- Lingler N and Karia S. Using AI to accelerate clinical trials. Deloitte. 2022 February. Accessed 18 August 2022. https://www2.deloitte.com/us/en/blog/health-care-blog/2022/using-ai-to-accelerate-clinical-trials.html.
- Mcgrail S. AI in the pharma industry: current uses, best cases, digital future. Pharmanews Intelligence. 2021 April. Accessed 21 August 2022. https://pharmanewsintel.com/news/ai-in-the-pharma-industry-current-uses-best-cases-digital-future#:~:text=AI%20has%20a%20great%20potential,during%20the%20COVID%2D19%20pandemic.
- Holzinger A, Langs G, Denk H, et al. Causability and explainability of artificial intelligence in medicine. 2019 April. Accessed 18 August 2022. doi: https://doi.org/10.1002/widm.1312
- Rossello J. Machine learning and pharmacovigilance. Pharmacovigilance analytics. 2022 March. Accessed 18 August 2022. https://www.pharmacovigilanceanalytics.com/methods/artificial-intelligence/machine-learning-and-pharmacovigilance/.
- Gupta R, Srivastava D, Sahu M, et al. Artificial intelligence to deep learning: machine intelligence approach for drug discovery. Mol Divers. 2021;25(3):1315-1360. doi:10.1007/s11030-021-10217-3.
- IBM Cloud Education. Unsupervised learning. 2020 September. Accessed 18 August 2022. https://www.ibm.com/cloud/learn/unsupervised-learning
- Bajaj V. Unsupervised learning in anomaly detection. Towards Data Science. 2020 August. Accessed 18 August 2022. https://towardsdatascience.com/unsupervised-learning-for-anomaly-detection-44c55a96b8c1
- Osiński B and Budek L. What is reinforcement learning? The complete guide. July 2018. Accessed 19 August 2022. https://deepsense.ai/what-is-reinforcement-learning-the-complete-guide/
- Aronson JK. Artificial Intelligence in pharmacovigilance: an introduction to terms, concepts, applications, and limitations. Drug Saf. 2022;45(5):407-418. doi:10.1007/s40264-022-01156-5
- Basile AO, Yahi A, and Tatonetti NP. Artificial intelligence for drug toxicity and safety. Trends Pharmacol Sci. 2019;40(9):624-635. doi:10.1016/j.tips.2019.07.005.
- Owczarek D. Augmenting drug safety and pharmacovigilance services with artificial intelligence (AI). Nexocode. April 2021. Accessed. 19 August 2022. https://nexocode.com/blog/posts/artificial-intelligence-for-pharmacovigilance/.
