Rapid COVID immune status test launched in UK, Ireland
pharmaphorum
JULY 26, 2021
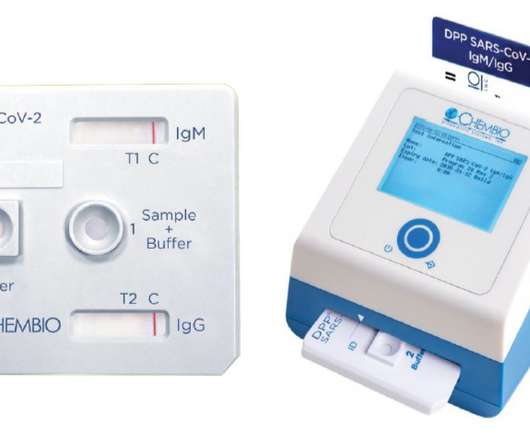
You’ve had your two COVID-19 jabs, but are you actually protected against infection? That’s a question that a fingerprick test launched today in the UK and Ireland could help to answer. The test – originally developed by US manufacturer Chembio Diagnostics – has been introduced into the UK and Irish markets by Guilford-based Luas Diagnostics and tests for the presence of SARS-CoV-2-targeting antibodies in the blood.


































Let's personalize your content