iRhythm digital heart monitoring service backed by NICE
pharmaphorum
DECEMBER 3, 2020
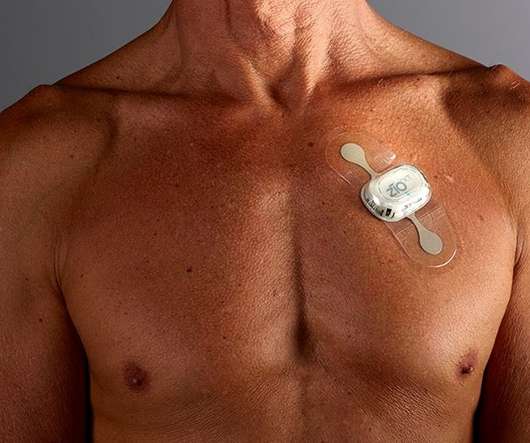
A heart monitor developed by iRhythm has become the first product to be endorsed by NICE in a pilot project covering digital health technologies. In new guidance, the health technology assessment (HTA) agency has recommended iRhythm’s Zio XT service for detecting abnormal heart rhythms – provided NHS organisations that deploy it collect evidence of its benefits.
































Let's personalize your content