Can machine learning accelerate drug formulation?
Outsourcing Pharma
JANUARY 24, 2023
Research emerging from the University of Toronto found that using machine learning models was able to guide the design of long-acting drug formulations.

Outsourcing Pharma
JANUARY 24, 2023
Research emerging from the University of Toronto found that using machine learning models was able to guide the design of long-acting drug formulations.
Pharmaceutical Technology
JANUARY 22, 2023
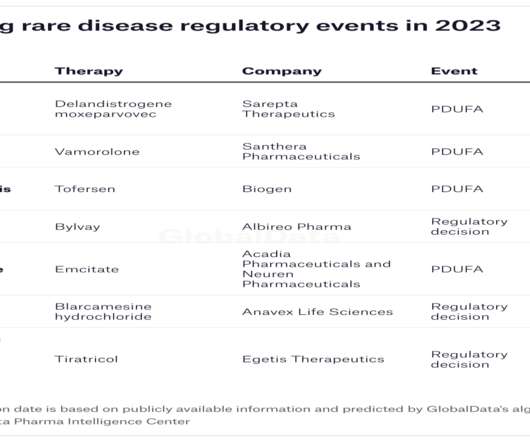
In 2022, the FDA approved only 37 new medicines, an underwhelming number compared to 98 in 2018. However, while only around 34% of the approvals in 2018 were for orphan drugs, 54% new approvals in 2022 were for drugs to treat rare diseases. Major pharmaceutical acquisitions have taken place in recent months in the rare disease space, as the number of orphan drug approvals continues to grow.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Fierce Healthcare
JANUARY 23, 2023
Amazon Pharmacy rolls out service for unlimited generic drug prescriptions for $5 a month hlandi Mon, 01/23/2023 - 22:15

PhRMA
JANUARY 26, 2023
Mental illness represents a broad spectrum of health conditions affecting mood, thinking and behavior. These conditions include depression, bipolar disorder, schizophrenia, substance use disorder, anxiety disorders, eating disorders, obsessive compulsive disorder and post-traumatic stress disorder, among others. Each of these illnesses often varies in its degree of severity, ranging from mild to moderate to severe, and may occur together.

Speaker: Chris Antypas and Josh Halladay
Access to limited distribution drugs and payer contracts are key to pharmacy expansion. But how do you prepare your operations to take the next step? Meaningful data: Collect and share clinical data regarding outcomes, utilization, and more Reporting: Limited distribution models require efficient tracking and reporting systems Workflows: Align workflows with specific pharma and payer contractual requirements For in-depth, expert insights on pharmacy expansion, watch this webinar from Inovalon.
Drug Topics
JANUARY 26, 2023
Spending on specialty drugs fueled a 11% increase in Medicaid drug spending from 2020 to 2021, says Magellan's seventh annual Medicaid Pharmacy Trend Report.

STAT
JANUARY 27, 2023
After weeks of deliberation, Pfizer was scolded by a U.K. pharmaceutical industry trade group after its chief executive officer made misleading statements in a media interview about the need to vaccinate young children against Covid-19. The fracas began when the Pfizer chief, Albert Bourla, gave an interview to the BBC and discussed the idea of vaccinating children between five and 11 years old, a course of action that had not yet been approved by regulators in the U.K.
Pharmacy Technician Pulse brings together the best content for pharmacy technicians from the widest variety of industry thought leaders.

PhRMA
JANUARY 25, 2023
Earlier this month, the American Cancer Society (ACS) released its annual report, Cancer Statistics 2023, finding the overall cancer mortality rate has declined 33% since peaking in 1991, leading to an estimated 3.8 million averted cancer deaths over this period. The report notes the continued decline in death rates since 1991, including a 1.5% decrease between 2019 and 2020 alone, increasingly reflects advances in prevention and treatment—including new medicines which have improved survival acr

Drug Topics
JANUARY 23, 2023
Community pharmacists need more support and resources to provide quality contraceptive counseling and care, according to a study published in BMJ Sexual and Reproductive Health.
STAT
JANUARY 26, 2023
Fasten your seat belts, folks. We’re about to hit some turbulence. If you’re reading this, you’re interested in the discussion on the future of Covid-19 vaccination that’s going to take place today in a meeting of the Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee. We at STAT can’t predict the outcome, but we know enough to expect that this meeting will feature some heated debate.

pharmaphorum
JANUARY 27, 2023
AstraZeneca’s revenue boost from COVID-19 therapy Evusheld looks set to be curbed early, as the FDA withdraws authorisation for the antibody on the grounds that it is ineffective against most subvariants now circulating in the US. Evusheld (tixagevimab and cilgavimab) was cleared by the FDA towards the end of 2021, becoming the first antibody to be authorised for prevention of COVID-19 infection, and it rapidly found use among people with compromised immune systems, such as cancer chemothe

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.

PhRMA
JANUARY 23, 2023
2022 marked another year of significant progress by biopharmaceutical research companies conducting lifesaving research and development for innovative medicines with a total of 45 new medicine approvals by the U.S. Food and Drug Administration (FDA). According to a new report released by the FDA’s Center for Drug Evaluation and Research (CDER), 37 novel medicines were approved by CDER last year.

Drug Topics
JANUARY 23, 2023
There may not be a diagnosis for ‘New Year Depression,’ but about 10% to 20% of individuals in the US may get a milder form of these winter blues.
STAT
JANUARY 23, 2023
Scientists at the Food and Drug Administration propose making Covid vaccination a regular, once-a-year shot that is updated to match current strains of the SARS-CoV-2 virus, according to documents posted by the FDA on Monday. For people who are older or immunocompromised, the FDA would recommend two annual doses of the revised shot.

pharmaphorum
JANUARY 26, 2023
2022 was a banner year for genomics. In March, the collaborative T2T consortium published the first complete telomere-to-telomere sequence of the human genome, filling in the last 8% of the 3 billion base pairs that make up our DNA. And in the UK specifically, genomics remained high on the national agenda, with several significant government programmes and investments announced – including the Newborn Genomes Programme in healthcare and the Precision Breeding Bill in the agricultural sector.

Advertisement
Are you still using workarounds to manage your daily operations? To achieve peak performance, it's time to explore other options for specialty and infusion pharmacy software. Streamline pharmacy operations and improve clinical performance with automated processing, real-time data exchange, and electronic decision support. Download this helpful infographic to: Drive efficiency and patient adherence from referral receipt to delivery and ongoing care – all with our Pharmacy Cloud.
European Pharmaceutical Review
JANUARY 23, 2023
The US Food and Drug Administration (FDA) has granted fast track designation (FTD) for Evaxion Biotech’s personalised cancer immunotherapy. The FTD is for EVX-01, in combination with Keytruda ® for patients with metastatic melanoma (MM). ”We are extremely pleased that our cancer vaccine candidate EVX-01 has received the FDA fast track designation, as it enables a potentially faster approval of the vaccine,” stated Per Norlén, CEO at Denmark-based Evaxion.
Drug Topics
JANUARY 27, 2023
Though, unvaccinated people are at much greater risk for severe outcomes than folks the same age who are caught up on boosters.

STAT
JANUARY 27, 2023
When institutions in the United States and other high-income countries embark on collaborations to improve health or the delivery of health care in low-income countries, they do it with the best of intentions. But intentions aren’t good enough. Projects conducted by trainees at schools of medicine, public health, and other health disciplines in high-income countries can often make the problems they set out to address worse.
Pharmaceutical Technology
JANUARY 23, 2023
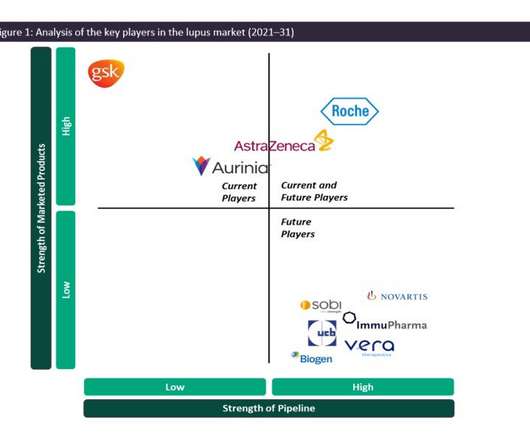
Systemic lupus erythematosus (SLE) is a systemic, inflammatory, chronic autoimmune disease that can affect multiple organs simultaneously or sequentially, with a relapsing and remitting nature. While SLE can affect multiple major organ systems in the body, one of its most severe manifestations is renal (kidney) involvement, known as lupus nephritis (LN).
European Pharmaceutical Review
JANUARY 26, 2023
Yescarta ® ▼(axicabtagene ciloleucel; axi-cel) is now the first chimeric antigen receptor (CAR) T-cell therapy and first personalised immunotherapy to be recommended for routine use on the NHS in England. This is based on final draft guidance from the National Institute for Health and Care Excellence (NICE). The therapy by Gilead Sciences and Kite is indicated for eligible adults with diffuse large B-cell lymphoma (DLBCL) and primary mediastinal large B-cell lymphoma (PMBCL) who have already bee

Drug Topics
JANUARY 27, 2023
A study aims to quantify the burden of current childhood asthma in the United States in association with smoke from indoor gas stoves.

STAT
JANUARY 26, 2023
WASHINGTON — The FDA is giving up on trying to figure out a way to regulate CBD on its own. The agency announced Thursday that it is formally calling on Congress for help — and, according to one official, looking for guidance on other hemp products like Delta 8 THC, too. For nearly four years, the Food and Drug Administration has been laboring to craft a solution that would allow CBD to be legally sold in capsules, gummy vitamins, and various foods, even though the agency also cons

Fierce Pharma
JANUARY 26, 2023
BMS settles lawsuit with two fired employees who refused COVID vaccines kdunleavy Thu, 01/26/2023 - 14:30

European Pharmaceutical Review
JANUARY 23, 2023
There are major concerns about microbial contamination in cannabis, US Food and Drug Administration (FDA) researchers observed in a study. Multiple cases have been reported of infections associated with cannabis use caused by fungi and bacteria in immunocompromised individuals using inhaled cannabis material. Investigational New Drug (IND) applications for cannabis as therapeutics tested in clinical trials must comply with FDA’s requirements and standards for drug products.

Drug Topics
JANUARY 25, 2023
Mallya can provide convenience for insulin pen users; however, it has yet to be released in the United States.
Fierce Healthcare
JANUARY 25, 2023
COVID-19 caused surge in heart disease deaths in first year of pandemic: report fdiamond Wed, 01/25/2023 - 08:44
pharmaphorum
JANUARY 27, 2023
The emergence of COVID-19 resulted in staffing challenges that have continued to have a sustained, negative impact on clinical trial workflows. In fact, 76% of healthcare professionals have recently cited feelings of burnout, leading to a turnover rate twice as high as before the pandemic and increased levels of vacant positions across clinical research sites.
STAT
JANUARY 23, 2023
Hospitals have bemoaned rising employee expenses throughout the pandemic, as they’ve paid workers more to prevent them from jumping to competitors, pursuing traveling gigs, or leaving the profession completely. But some, like those in Texas, have been able to bring in traveling nurses and other temporary staff on the taxpayers’ dime.
Drug Topics
JANUARY 27, 2023
Recommendations from the unanimous vote must be adopted by the FDA and CDC.
Fierce Healthcare
JANUARY 27, 2023
SDOH startup Spatially Health partners with Florida ACO amid new CMS rules on equity agliadkovskaya Fri, 01/27/2023 - 13:40

pharmaphorum
JANUARY 27, 2023
Late last year, pharmaphorum caught up with Dr Karen Mullen, chief medical officer and VP of clinical & medical affairs at global drug development consultancy Boyds. Having previously spoken with Dr Mullen three years ago when she was country medical director for the UK & Ireland at GSK – on the topic of patient centricity and embedding that focus within the core of a pharmaceutical company – our recent interview provided some interesting insights into the changing landscape of the indus

STAT
JANUARY 26, 2023
A loophole in prescription drug regulation exposes American consumers to false and misleading claims of drug ads via on-line businesses promoting potent drugs without accurate information about their risks and benefits. Many countries prohibit direct-to-consumer advertising of prescription drugs. The United States allows it, but only if pharmaceutical companies comply with standards for accuracy and balance established and enforced by the Food and Drug Administration.
Pharmaceutical Technology
JANUARY 22, 2023
Eli Lilly and Company and Boehringer Ingelheim have announced that the US Food and Drug Administration (FDA) accepted a supplemental New Drug Application (sNDA) for Jardiance (empagliflozin) tablets for chronic kidney disease (CKD) in adult patients. Jardiance is currently being evaluated as a potential therapy for reducing kidney disease progression and cardiovascular death risk in CKD adult patients.

Drug Store News
JANUARY 24, 2023
RxPass is a new Prime membership benefit from Amazon Pharmacy offering patients affordable access to commonly prescribed generic medications for more than 80 common health conditions.

pharmaphorum
JANUARY 27, 2023
Johnson & Johnson has been cleared to continue a lawsuit filed last year against drug benefit programme SaveOnSP, which claims it defrauded a payment assistance programme for patients out of “at least $100 million.” The complaint – originally filed in May 2022 in a New Jersey federal court – alleges that SaveOnSP took advantage of J&J’s Janssen CarePath programme, which covers eligible patients’ out-of-pocket expenses for 44 of its more expensive prescription drug
Let's personalize your content