Breast Cancer Research, Current Therapeutic Pipeline Provide Significant Advancements in Diagnosis, Treatment
Trends in treatment development involve more targeted, safe, and efficacious therapies.
Over the past several years, researchers have commanded significant innovation to improve the diagnosis, treatment, and prognosis for patients with breast cancer. Notable developments in early-stage breast cancer are improving outcomes by matching the right individualized treatment for patients to obtain the most benefit.1,2 Different preventive measures and treatment modalities are being studied, including advancements in screening techniques, modifiable risk factors, enhanced surgical options, and novel therapeutics uniquely targeting the disease.
Breast cancer is the most common type of cancer, accounting for 15% of all new cancer diagnoses.1,2 In the United States, more than 290,000 new cases are expected in 2022, with more than 43,000 estimated deaths.1,2 When included as a member of oncology patient care teams, specialty pharmacists are not only uniquely positioned to provide patients with advice on managing medications, but also can encourage screening, increase adherence, offer in-depth disease state education, and connect patients and caregivers with
valuable resources.
Basics in Understanding Breast Cancer
Breast cancer begins when cells in 1 or both breasts begin to grow out of control, and it spreads by moving into the blood or lymphatic system and is then carried throughout the body. Although breast cancer occurs most often in women, men can also be diagnosed with the disease.3,4
Signs of breast cancer include changes in the size or shape of the breast, scaling, redness, swelling, dimpling or puckering in the skin, an inverted nipple, fluid other than breast milk from the nipple, or a lump in or near the breast or underarm area. Tests that examine the breasts are used to screen for cancer, including a clinical breast exam, mammogram, ultrasound, MRI, blood chemistry, and biopsy.3,4
Several known risk factors may contribute to the development of breast cancer. Modifiable risk factors include physical activity level, weight, alcohol consumption, exogenous hormones, and certain aspects of a woman’s reproductive history, such as age at first pregnancy and breastfeeding. Nonmodifiable risk factors include older age, genetic mutations, age at onset of menstruation and menopause, breast density, personal or family history of breast or ovarian cancer or certain noncancerous breast diseases, previous radiation therapy, and exposure to certain chemicals, such as the drug diethylstilbestrol.3,4,5
Among women with breast cancer, less than 15% have a first-degree relative with the disease.6 Breast cancer can be caused by inherited mutations to the BRCA1 or BRCA2 tumor suppressor genes, but these mutations are uncommon, with an overall rate in the United States of approximately 1 in 400.7 There are many different possible mutations of BRCA1/2, and some increase the risk for cancer. The lifetime risk of breast cancer for women in the United States who do not have a BRCA1/2 mutation is 13%.7,8 With certain BRCA1/2 mutations, this risk increases to up to 75%.8
Different types of breast cancer are determined by the specific kinds of cells affected and are categorized by certain types of genes or proteins the cancer cells may generate, which are differentiated as hormone receptor–positive (HR+) or HR negative based on whether they have these receptors.3,4 HR+ breast cancer cells have either estrogen receptors (ER+), progesterone receptors, or both.3,4
Approximately 15% to 20% of breast tumors have higher levels of HER2 protein, which helps breast cancer cells grow rapidly.9 Cancer cells with no to very low levels of ERs, progesterone receptors, and HER2 are called triple-negative breast cancer (TNBC). Knowing the HR status of the cancer allows the health care team to best determine how to approach treatment. Additionally, tumor cells are reviewed to determine both the grade and stage of the cancer; this information is then used to help guide decisions regarding treatment options.
The stage of the cancer defines how large the cancer is and whether it has spread, whereas grading determines how abnormal the cancer cells appear under a microscope.4 In general, the lower the grade number, the slower growing and less likely the cancer is to spread.3,4
Current Treatment Options
A variety of therapies and a spectrum of medication classes, including biosimilars, are available to treat the many different types of breast cancer. Further, treatment guidelines continue to evolve, and selection depends on variables including tumor biology and molecular identity, HR+/– status, HER2 expression, tumor size, disease stage, nodal involvement, menopausal status, other comorbidities, and patient preferences.10
Today, the 6 types of standard treatment used are surgery, radiation, chemotherapy, hormone therapy, targeted therapy, and immunotherapy; patients with early-stage breast cancer typically undergo surgery, followed by adjuvant treatment.3,4 However, for patients with advanced or metastatic breast cancer (mBC), the disease is usually treated with medication because surgical intervention is no longer an option. In certain cases, surgery may be an option used in the palliative care setting.3,4
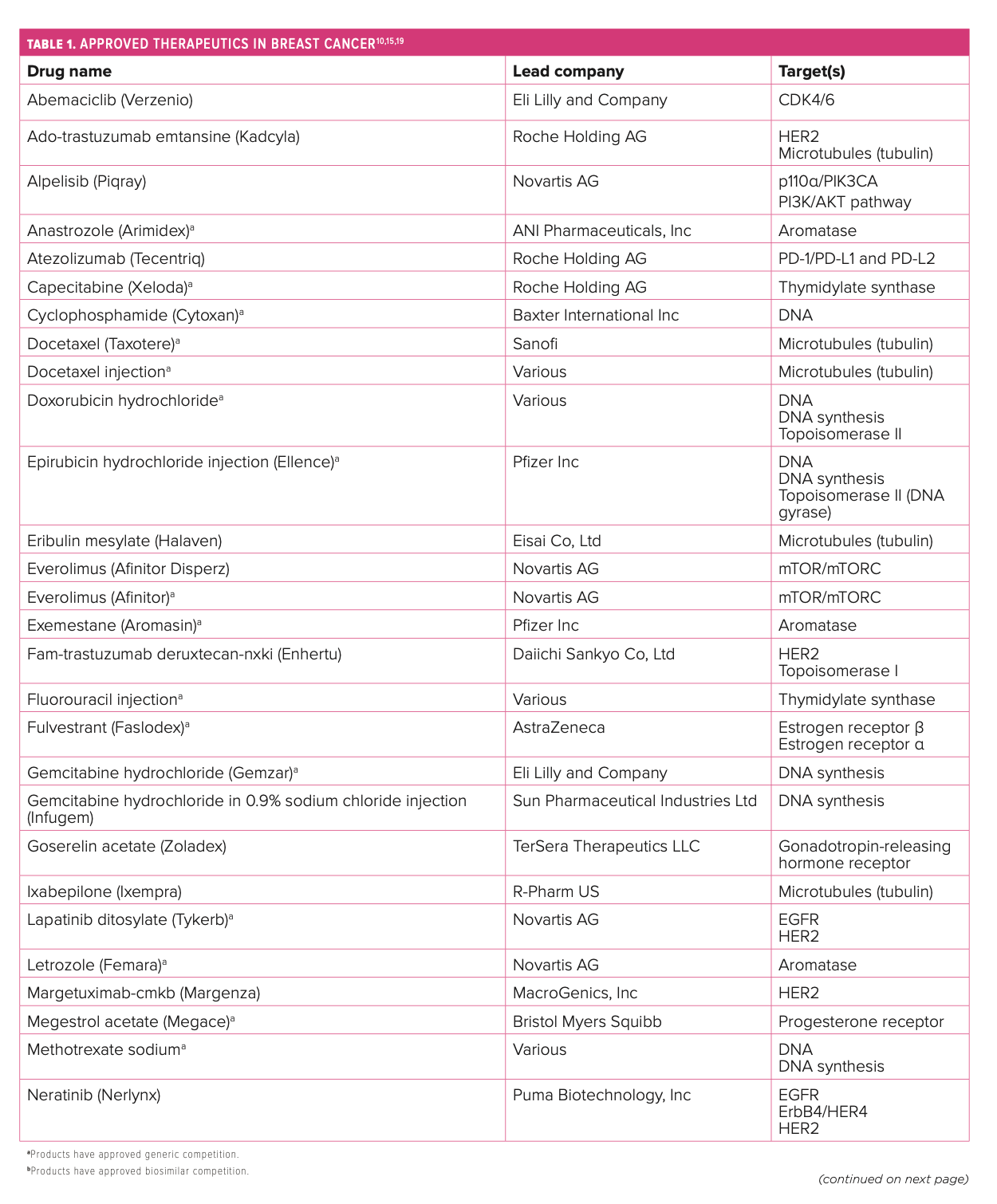
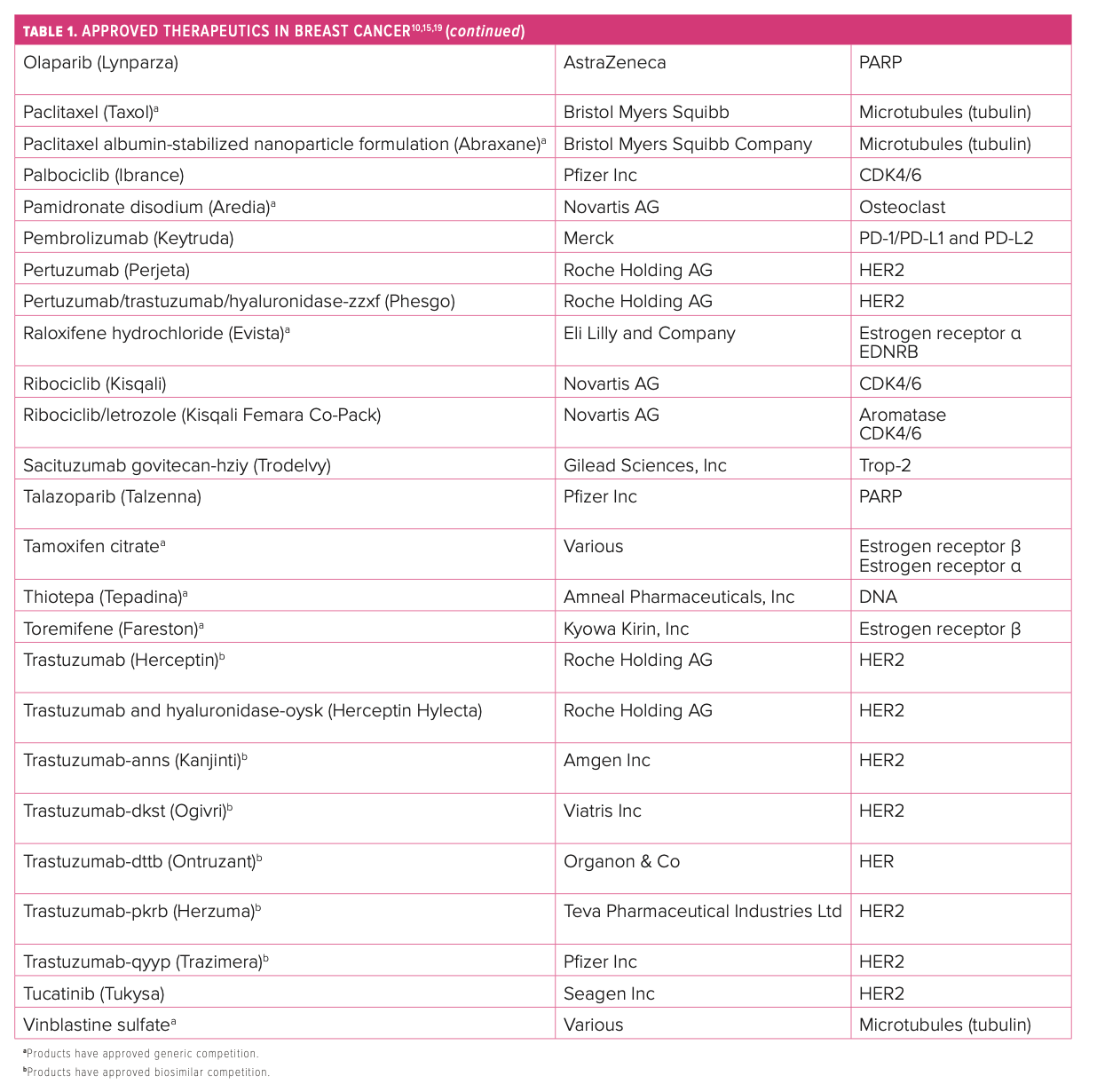
Several specific molecular targets represent the current armamentarium for tackling the disease. Because of its cytotoxic nature, traditional chemotherapy often comes with off-target adverse effects (AEs), but these regimens still have a role in treatment as monotherapy or in combinations. When started early, HR regimens are often as effective as traditional chemotherapy options and reduce the likelihood of experiencing many AEs.11 HER2 has been successfully targeted with the combination of pertuzumab (Perjeta; Roche Holding AG) plus trastuzumab (Herceptin; Roche Holding AG) as well as margetuximab-cmkb (Margenza; MacroGenics, Inc) for certain patients, and more cytotoxic drug conjugates are making progress in clinical trials.12
PARP inhibitors combat breast cancer that expresses germline BRCA1/2 mutations.13 The PD-1/PD-L1 pathway is another area of focus in the immunotherapy space when brain metastases may be involved.14 CDK4/6 inhibitors have been established as the standard of care in HR+/HER2-negative (HER2–) mBC but with no clear preference regarding which should be used first line.15,16 Blocking the PI3K signaling pathway combats PIK3CA-mutated tumors of HR+ mBC.17 Tyrosine kinase inhibitors play a role in fighting HER2+ mBC and may protect against brain metastases.18 The first monoclonal antibody targeting Trop-2, sacituzumab govitecan-hziy (Trodelvy; Gilead Sciences, Inc), was approved in 2021 for the treatment of metastatic TNBC, and more agents are being studied in this novel class (Table 110,15,19).20
What to Watch for in Breast Cancer Therapeutics
The anticipated expansion of novel therapies in the near-term pipeline may continue to change the current standards of care in breast cancer and offer additional promising treatment options for patients. Several noteworthy updates have occurred in the field of breast cancer this year, particularly in developments for early-stage disease.
The 2022 American Society for Clinical Oncology (ASCO) Annual Meeting featured several important presentations, including21:
- tailoring adjuvant therapy;
- immunotherapy to improve response in patients with early-stage TNBC;
- tumor molecular profiling for predicting treatment response in patients with early-stage HER2+ disease;
- new tests to help identify patients with ER+ breast cancer who would be most likely to benefit from chemotherapy;
- the use of an osteoporosis drug to decrease bone fractures and improve outcomes in patients receiving aromatase inhibitor therapy;
- and commentary on older patients with early-stage breast cancer who may forgo chemotherapy.
A key treatment is fam-trastuzumab deruxtecan-nxki (Enhertu; Daiichi Sankyo Co, Ltd), which was recently approved based on the DESTINY-Breast03 clinical trial (NCT03529110) results for the treatment of adult patients with unresectable or HER2+ mBC who have received a prior anti–HER2-based regimen either in the metastatic setting or in the neoadjuvant or adjuvant setting and have developed disease recurrence during or within 6 months of completing therapy.10 Of note, fam-trastuzumab deruxtecan-nxki received a priority review and breakthrough therapy designation from the FDA and was also recently approved for the treatment of adult patients with unresectable or metastatic HER2-low breast cancer who have received a prior chemotherapy in the mBC setting or developed disease recurrence during or within 6 months of completing adjuvant chemotherapy.19
HER2 low is a newly defined subset of HER2–breast cancer in which there are some HER2 proteins on the cell surface but not enough to be classified
as HER2+.6,19 The phase 3 DESTINY-Breast04 trial (NCT03734029) results showed fam-trastuzumab deruxtecan-nxki reduced the risk of disease progression or death and improved median overall survival (OS) by more than 6 months versus chemotherapy.20 Fam-trastuzumab deruxtecan-nxki is the first HER2-directed therapy to demonstrate survival benefit in patients with HER2-low mBC compared with standard of care, and with the expanded approval, it has the potential to redefine treatment for approximately half of all patients with breast cancer.19
Another important takeaway from the ASCO meeting, based on the OlympiA clinical trial (NCT02032823), olaparib (Lynparza; AstraZeneca) was approved in 2022 for the adjuvant treatment of adult patients with deleterious or suspected deleterious germline BRCA-mutated HER2–high-risk early breast cancer who have been treated with neoadjuvant or adjuvant chemotherapy.10
CDK4/6 inhibitor agents as a class have demonstrated an increase in progression-free survival (PFS) in clinical trials, but more recent postmarketing trials have highlighted several differences in efficacy, including OS statistics.15,19 If proven effective, combining CDK4/6 agents with endocrine therapies against HR+ mBCs may facilitate avoiding traditional chemotherapy.16 Analysts predict that an uptake in ribociclib (Kisqali; Novartis AG) utilization may occur as the result of its clinically significant OS data and could pave the way for it to potentially become the preferred agent in this class and open the door for potential contracting.15,19 Further, the phase 3 HARMONIA trial (NCT05207709) is slated to assist in understanding optimal treatment approaches for patients with HER2-enriched disease, a subtype that is associated with very poor prognosis and endocrine resistance.15,19 Another phase 3 trial, NATALEE (NCT03701334), is scheduled to evaluate ribociclib as adjuvant treatment for HR+/HER2– stages 2 and 3 breast cancers (Table 210,19).15,19
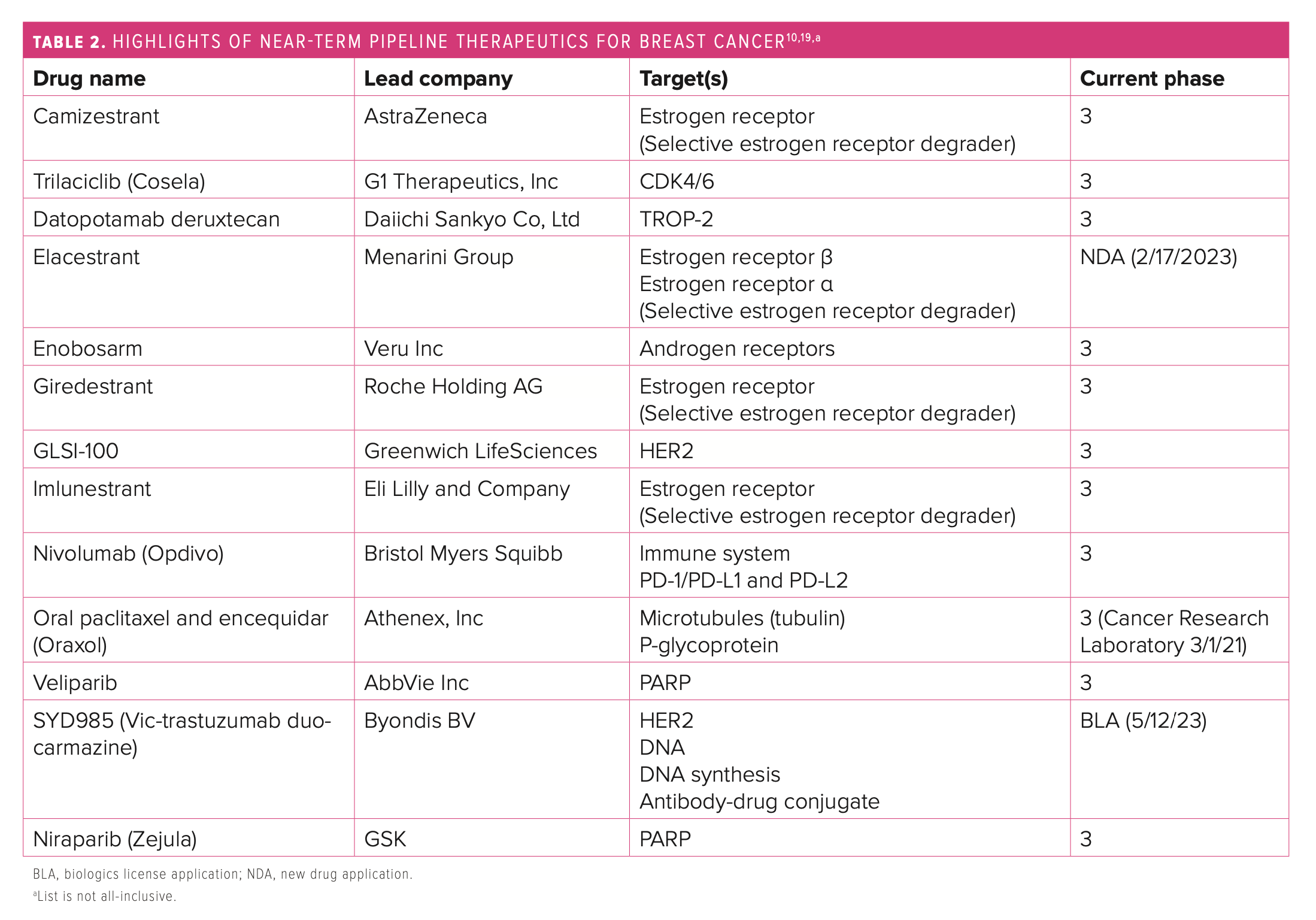
Of note, an oral formulation of paclitaxel (Oraxol; Athenex, Inc) in combination with encequidar, a gastrointestinal tract–specific P-glycoprotein pump inhibitor that allows the drug to be orally absorbed, is being studied in a phase 3 trial.10,15,19 The product was compared with intravenous paclitaxel in patients with mBC, including those with HR+/HER+ disease, TNBC, and receptor status unknown.10,15,19 The oral form demonstrated a higher overall response rate and was associated with lower rates of neuropathy but higher rates of neutropenia.10,15,19 Drawbacks to the product include administration, which requires fasting for 9 hours per day for 3 consecutive days per week, and higher gastrointestinal toxicities.10,15,19
Vic-trastuzumab duocarmazine is an investigational, next-generation anti-HER2 antibody-drug conjugate pursuing placement as treatment for third-line mBC and second line after ado-trastuzumab emtansine (Kadcyla; Roche Holding AG).10,15,20 The biologics license application was accepted for the treatment of patients with HER2+ unresectable, locally advanced mBC or mBC.10,15,19
Several oral selective estrogen receptor degrader (SERD) drugs are in development for patients with previously treated ER+/HER2– mBC.10,15,19 Their use is being investigated as second- and third-line options in postmenopausal women who have progressed with prior endocrine and targeted therapies, and studies suggest they may be most beneficial in ESR1-mutated tumors.10,15,19 Analysts estimate the market for SERDs could be worth at least $2 billion to $3 billion a year.15 Currently, elacestrant (Menarini Group and Radius Health, Inc) appears to have the most promising data from its phase 3 EMERALD trial (NCT03778931), meeting the primary end point of PFS.15 Giredestrant (Genentech, Inc) failed the primary end point of PFS in its acelERA Breast Cancer trial (NCT04576455), but coopERA Breast Cancer (NCT04436744) is the first randomized study to show superior antiproliferative activity of an oral SERD vs an aromatase inhibitor in ER+/HER2– early breast cancer.15,19 Studies are ongoing to further assess the product’s clinical benefit, and Roche plans to file for approval in second-line/ third-line mBC in 2022.19
Supporting Patients With Breast Cancer by Engaging the Specialty Pharmacist
At AllianceRx Walgreens Pharmacy, everything our specialty pharmacists do centers on the well-being of our patients. Being given a potentially life-altering diagnosis like breast cancer can be overwhelming, but starting a specialty medication shouldn’t be. Medication therapy management for patients involves education with drug-specific and disease-state counseling, emphasizing the importance of adherence to the prescribed regimen, guiding patients in preventing and managing AEs, and monitoring for any potential drug interactions.
Specialty pharmacists may also help point patients in the right direction for additional resources and are available to address any concerns or questions patients and caregivers may have regarding therapy. Because specialty drugs are often associated with higher costs, specialty pharmacies can help patients explore all options to find the most affordable care. This may include searching for various foundations and supporting patients in applying for co-pay assistance or other pharmacy-related financial assistance programs when available. Working together with other health care providers, specialty pharmacists can serve as therapeutic navigators helping to ensure patients achieve the most optimal outcomes, both clinically and financially.
Conclusion
Significant support for breast cancer awareness, research, and funding has paved the way for advancements in the diagnosis and treatment of the disease. Survival rates have increased, and the number of deaths associated with breast cancer is steadily declining, largely because of factors such as early detection, personalized approaches to treatment, and a better understanding of the disease overall.10,15,19 The growing trends in breast cancer treatment involve more targeted, safer, and efficacious therapies that better align with patient goals and outcomes. The advancements in research offer astounding opportunities focused on overall survival, historically treatment-resistant disease, and reducing disease progression.10,15,19 Analysts expect more effective oral options for HER2+ breast cancer, with de-escalation of chemotherapy or surgery as superior choices.10,15,19
Specialty pharmacists, such as those at AllianceRx Walgreens Pharmacy, understand treatment decisions are very personal, and patients should discuss all options with their providers in a shared decision-making process to agree on a treatment plan. As standards of practice continue to evolve, specialty pharmacists can help develop individualized management plans for patients with breast cancer and support them throughout their treatment journey. Additionally, a key focus for pharmacists moving forward in the battle against breast cancer is continuing to encourage patients to schedule breast cancer screenings because early detection plays a major role in successfully overcoming this disease.
About The Author
Rachel K. Anderson, PharmD, CSP, is the clinical program manager at AllianceRx Walgreens Pharmacy.
References
1. Common cancer types. National Cancer Institute. Updated May 10, 2022. Accessed July 27, 2022. www.cancer.gov/types/common-cancers#:~:text=The%20most%20common%20type%20of,are%20combined%20for%20the%20list
2. Cancer stat facts: female breast cancer. National Cancer Institute: Surveillance, Epidemiology, and End Results Program. 2022. Accessed July 27, 2022. seer.cancer.gov/statfacts/html/breast.html
3. Breast Cancer Prevention (PDQ)–Patient Version. National Cancer Institute. Updated December 10, 2021. Accessed July 27, 2022. www.cancer.gov/types/breast/patient/breast-prevention-pdq
4. Breast cancer. The American Cancer Society. Accessed July 27, 2022. www.cancer.org/cancer/breast-cancer.html
5. What are the risk factors for breast cancer? CDC. Updated Sept 26, 2022. Accessed July 27, 2022. www.cdc.gov/cancer/breast/basic_info/risk_factors.htm
6. Lynch JA, Venne V, Berse B. Genetic tests to identify risk for breast cancer. Semin Oncol Nurs. 2015;31(2):100-107. doi:10.1016/j.soncn.2015.02.007
7. BRCA1 and BRCA2 inherited gene mutations in women. Susan G. Komen. Updated March 21, 2022. Accessed September 16, 2022. https://www.komen.org/breast-cancer/risk-factor/topics/brca-genes
8. BRCA overview. Basser Center for BRCA. Accessed September 16, 2022. https://www.basser.org/brca
9. Breast cancer hormone receptor status. American Cancer Society. Updated November 8, 2021. Accessed July 27, 2022. www.cancer.org/cancer/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-hormone-receptor-status.html
10. Oncology: breast cancer. IPD Analytics. Accessed July 27, 2022. secure.ipdanalytics.com/User/Pharma/RxStrategy/Page/bfbb1763-919a-4303-b614-8cdce426b48f#section-group-332627
11. Martí C, Sánchez-Méndez JI. The present and future of neoadjuvant endocrine therapy for breast cancer treatment. Cancers (Basel). 2021;13(11):2538. doi:10.3390/cancers13112538
12. FDA approves margetuximab for metastatic HER2-positive breast cancer. FDA. Updated December 17, 2020. Accessed July 27, 2022. www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-margetuximab-metastatic-her2-positive-breast-cancer
13. Tung NM, Robson ME, Ventz S, et al. TBCRC 048: phase II study of olaparib for metastatic breast cancer and mutations in homologous recombination-related genes. J Clin Oncol. 2020;38(36):4274-4282. doi:10.1200/jco.20.02151
14. Kim JS, Kim IA. Evolving treatment strategies of brain metastases from breast cancer: current status and future direction. Ther Adv Med Oncol. 2020;12:1758835920936117. doi:10.1177/1758835920936117
15. IPD Analytics. Strategy reports: the evolving landscape of CDK 4/6 inhibitors in metastatic breast cancer. July 25, 2022. Accessed July 27, 2022. secure.ipdanalytics.com.IPDAnalytics_RxInsights_Evolving Landscape of CDK 4 and 6 Inhibitors in Metastatic Breast Cancer_07 2022.pdf
16. Tolaney SM, Wardley AM, Zambelli S, et al. Abemaciclib plus trastuzumab with or without fulvestrant versus trastuzumab plus standard-of-care chemotherapy in women with hormone receptor-positive, HER2-positive advanced breast cancer (monarcHER): a randomised, open-label, phase 2 trial. Lancet Oncol. 2020;21(6):763-775. doi:10.1016/s1470-2045(20)30112-1
17. Piqray. Prescribing Information. Novartis Pharmaceuticals Corporation; May 2022. Accessed July 27, 2022. www.novartis.com/us-en/sites/novartis_us/files/piqray.pdf
18. Garcia-Alvarez A, Papakonstantinou A, Oliveira M. Brain metastases in HER2-positive breast cancer: current and novel treatment strategies. Cancers (Basel). 2021;13(12):2927. doi:10.3390/cancers13122927
19. FDA grants regular approval to sacituzumab govitecan for triple-negative breast cancer. FDA. Updated April 8, 2021. Accessed July 27, 2022. www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-sacituzumab-govitecan-triple-negative-breast-cancer
20. Biomedtracker. Informa Pharma Intelligence. Accessed September 6, 2022. biomedtracker.com
21. ASCO 2022: early-stage breast cancer updates. Breast Cancer Research Foundation. July 15, 2022. Accessed July 27, 2022. www.bcrf.org/blog/asco-2022-early-stage-breast-cancer-research-updates-bcrf/

