Survey: industry leaders looking to shore up pharma supply chain
Outsourcing Pharma
MAY 5, 2022
According to the 2022 EY CEO survey, life science executives are seeking to build resilience into their supply chains and dive into M&A opportunities.

Outsourcing Pharma
MAY 5, 2022
According to the 2022 EY CEO survey, life science executives are seeking to build resilience into their supply chains and dive into M&A opportunities.
pharmaphorum
MAY 5, 2022
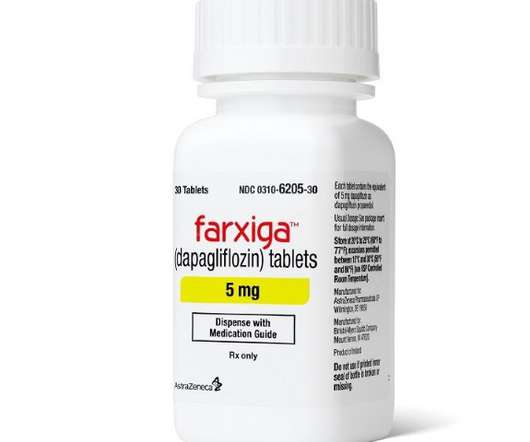
AstraZeneca’s SGLT2 inhibitor Farxiga has hit the mark in a phase 3 heart failure trial that brings it back into contention with its main rival Jardiance from Boehringer Ingelheim and Eli Lilly. Top-line results from the DELIVER trial showed that Farxiga (dapagliflozin) was able to reduce the risk of cardiovascular deaths or worsening heart failure in patients who have heart failure with preserved ejection fraction (HFpEF), setting up filings for the new indication in the coming months.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Outsourcing Pharma
MAY 5, 2022
A leader from the clinical technology company discusses some of the pain points in data collection and analysis, and tools that can help users level up.
pharmaphorum
MAY 5, 2022
Three years after it was formed as a spinout of Japanese drugmaker Takeda, Phathom Pharmaceuticals has racked up its first FDA approvals, and claimed a sizeable $260 million in new financing to help with the rollout. The US regulator has cleared two therapies based on Phathom’s acid blocker vonoprazan in combination with antibiotics for Helicobacter pylori infections , which are associated with peptic ulcer disease and some forms of gastric cancer, with launches scheduled for the third qua

Speaker: Chris Antypas and Josh Halladay
Access to limited distribution drugs and payer contracts are key to pharmacy expansion. But how do you prepare your operations to take the next step? Meaningful data: Collect and share clinical data regarding outcomes, utilization, and more Reporting: Limited distribution models require efficient tracking and reporting systems Workflows: Align workflows with specific pharma and payer contractual requirements For in-depth, expert insights on pharmacy expansion, watch this webinar from Inovalon.

Outsourcing Pharma
MAY 5, 2022
This monthâs news on partnerships, appointments, expansions, and investment includes Javara, ACG, Phastar, Elligo Health Research, and other notable companies.

pharmaphorum
MAY 5, 2022
SAE Media Group’s 3rd Annual. Wearable Injectors and Connected Devices Conference. 10 – 11 October 2022 | London, UK. [link]. The Latest Innovations In Digital Health And On-Body Drug Delivery For The Pharmaceutical Industry. SAE Media Group’s 3rd annual Wearable Injectors and Connected Devices Conference will explore on-body device design and development whilst also engaging in the latest advances in connectivity and digital health applications for wearable devices with industry perspecti
Pharmacy Technician Pulse brings together the best content for pharmacy technicians from the widest variety of industry thought leaders.

pharmaphorum
MAY 5, 2022
Assuring end-patient safety through holistic contamination control. Sponsored by: Associates of Cape Cod, Bioscience International and Ecolab. Conference Chairman: Don Singer, USP General Chapter Committee, Microbiology Chair, US Pharmacopeia and ECOLAB. Following on the success of the previous West Coast events, we are delighted to announce 5th Annual Pharmaceutical Microbiology West Coast conference taking place in San Diego on June 20th and 21st,2022.

Pharma Times
MAY 5, 2022
Jakavi treats a potentially life-threatening condition which often effects transplant patients

pharmaphorum
MAY 5, 2022
Preparation and practice are potent forces in sales but they form a golden triangle when combined with feedback. Shaping performance with guidance elevates coaching to new heights. Following scripted guidelines will only get you so far along the route to high quality results, according to sales and field force exports. Academic and field research has shown that feedback reinforces good practise, encourages the adoption of new skills and motivates staff to chase higher levels of performance.
pharmaphorum
MAY 5, 2022
Sidekick Health is already working with pharma heavyweights Pfizer and Bayer on gamified apps that can be paired with drug therapies to improve patient outcomes – and says it is poised to announce three more in the coming months. The Iceland-based digital health company – which has just raised $55 million in second-round funding – has been working with Bayer on an app/medicine combination for patients with peripheral artery disease since 2019, and in the following year cut a deal with Pfizer to

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.

pharmaphorum
MAY 5, 2022
Curebase has raised $40 million in second-round financing that will help it run more complex interventional trials for its biopharma clients, according to chief executive Tom Lemberg. The Series B takes the total raised by Curebase to $59 million since it was set up five years ago to develop a patient-focused approach to clinical research, though the development of a software platform initially aimed at running decentralised clinical trials (DCT).

pharmaphorum
MAY 5, 2022
After a long period of not much change, clinical trials are undergoing several massive shifts. At the American Telemedicine Association conference in Boston this week, a Sunday afternoon panel explored the intersection of two of these changes: the growing use of telehealth in decentralised clinical trials, and an increased awareness of the importance of health equity and diversity in trials.

pharmaphorum
MAY 5, 2022
A quick recap: Who are CDISC and what do they do? The Clinical Data Interchange Standards Consortium (CDISC) is a non-profit organization that aims to maximise the impact of clinical research data by establishing and promoting the use of data standards. Basically, these standards make clinical data easier to understand and interpret. “We develop and advance data standards of the highest quality to transform incompatible formats, inconsistent methodologies, and diverse perspectives into a power
Let's personalize your content