Important factors to consider when working with CRFs

What’s the background on CRFs?
A traditional paper case report form is known as a CRF (or paper CRF). An electronic case report form (eCRF) is the same as a CRF, except that it’s electronic. Both abbreviations tend to be used interchangeably, and are also referred to as forms.
CRFs and eCRFs are used for gathering patient data during clinical trials. They play a crucial role in helping to assess the safety and efficacy of clinical products.
For a study to be successful, data collected must be correct and complete. To be correct and complete, forms must be well planned with meticulous attention to detail. They must comply with the study protocol, and record its detail. They must also comply with regulatory requirements, such as those defined by the FDA.
CRF design & eCRF design
Good CRF Design is essential for a successful clinical trial submission and getting clinical products to the market.
Design objectives
Well-designed forms must:
- Gather data that’s complete, accurate, and of high quality.
- Avoid duplication.
- Be well structured and easy for the user to complete.
- Be unambiguous and allow for accurate data entry. For example, using coded lists to limit answers to questions. And avoid open-ended questions.
- Avoid gathering more data than what is needed.
- Be consistent, well laid out, uncluttered, simple, and user friendly.
What to keep in mind at design time
To capture data correctly, here’s some things to think about:
- Clear guidance using prompts and instructions should be included.
- Formats, fonts, and font sizes should be the same across all forms.
- The layout should be simple and uncluttered.
- Questions should be clear, precise, and easy to understand.
- Include consistent headers.
- Specify units of measurement.
- Visual cues should be used to show how questions are to be answered. For example, the date format, and the number of decimal places.
- Minimize the use of free-text responses or “check all that apply”.
- Keep related questions together in sections.
- For paper CRFs, include page numbering. Avoid circling answers, and make it clear which questions are mandatory.
Example of a well-designed form
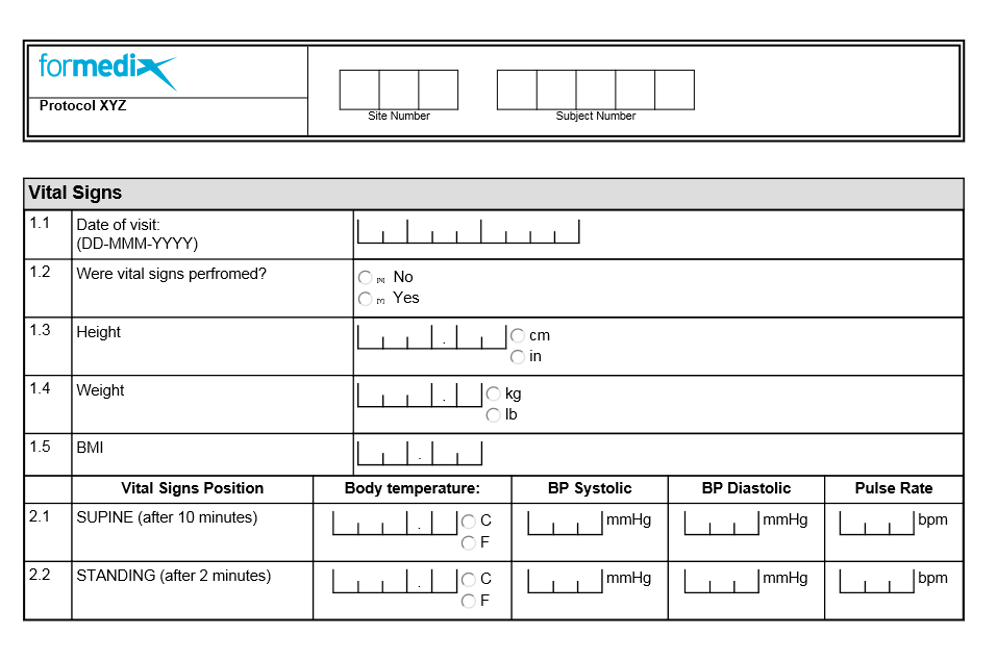
Clear guidance is given for each response in the form above, so there will be no unnecessary queries. The yes / no response is coded and should be coded in the same way across all forms in studies for consistency.
Example of a poorly designed form
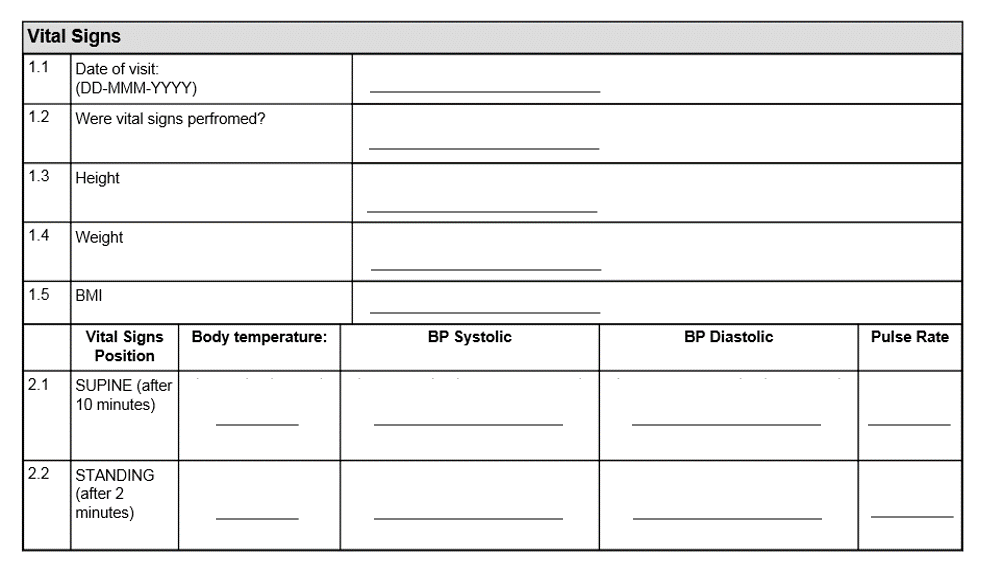
There’s no guidance given for responses in the form above. That means results will vary in responses from site to site, and from investigator to investigator.
CRF design & eCRF design challenges
3 common design challenges that typically come up:
- Creating forms that are consistent.
- Collecting precise data.
- Creating user-friendly forms.
To overcome these challenges there’s a number of things that can be done.
- Do proper planning, and start it early on in the study. This should be done by a team of people that include data management, biostatisticians, and clinicians.
- Define clear objectives and stick to them.
- Maintain standardized forms.
- Get user feedback. It’s best to build this into the design and maintenance process.
- Apply best practices.
- Provide form completion guidelines to reduce data capture and data entry issues. This helps investigators fill in forms correctly by providing step by step guidance, and uses clear specific instructions.
That's CRF and eCRF design covered, the next thing to look at is annotating CRFs.
Why are annotated CRFs so important?
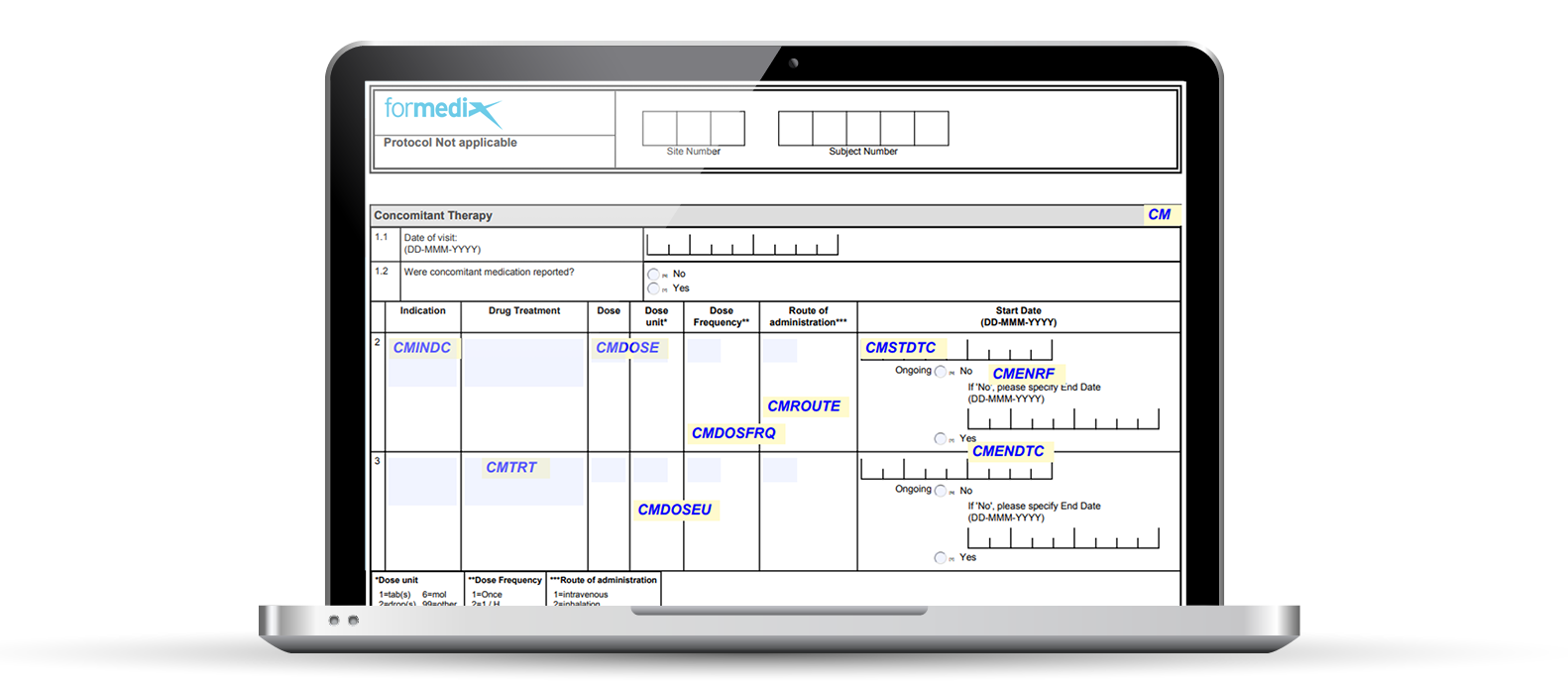
Annotated CRFs are a key submission deliverable, a mandatory requirement of the FDA. Each form in a study contains markings or annotations. These annotations map data points on forms to the name of datasets, and variables within those datasets. In other words, “each CRF should provide the variable names and coding for each CRF item included in the data tabulation datasets” as stated by the FDA guidelines.
The FDA stipulates that a set of blank annotated CRFs be submitted in a PDF document called “blankcrf.pdf”. This document helps the FDA reviewer find the origin of variables in the SDTM datasets.
You can read more about why it’s a good idea to switch to automated CRF annotations.
Here’s an example of an annotated CRF in Formedix.
Why should CRFs be standardized?
It’s important to standardize forms so that stakeholders such as the investigator, data manager, biostatisticians, data entry personnel, etc. have their needs met. CRFs must be user friendly and capture consistent data, that’s clear and valid.
Standardizing CRFs means you can reuse them. That’s a huge efficiency gain. They’ve already been reviewed and approved, so they’ll be consistent, and of high quality. And, annotations don’t need to be manually done on each form. You can also reuse edit checks that normally take lots of time and resources to do. So, not only is it a huge time and resource-saving, it’s a lot less hassle!
How Formedix can help
Our clinical trial automation software lets you create forms from scratch, or upload your existing forms and store them in our clinical metadata repository. Once your forms are standardized you can quickly and easily reuse them across other standards and studies. Including annotations and edit checks. It’s easy to find, share, and update your forms. And you can preview them in different formats as you design them, as well as being able to see how they look and work, in 7 leading EDCs including Rave and InForm. And CRF, EDC, and CDISC validation are built-in, so you don’t have to worry about being compliant. You can find more about creating your CRF designs and EDC designs in Formedix.
If you’ve found this interesting you can click on Everything you want to know about CRFs to read the full article. And you can visit the Formedix website at https://www.formedix.com/











