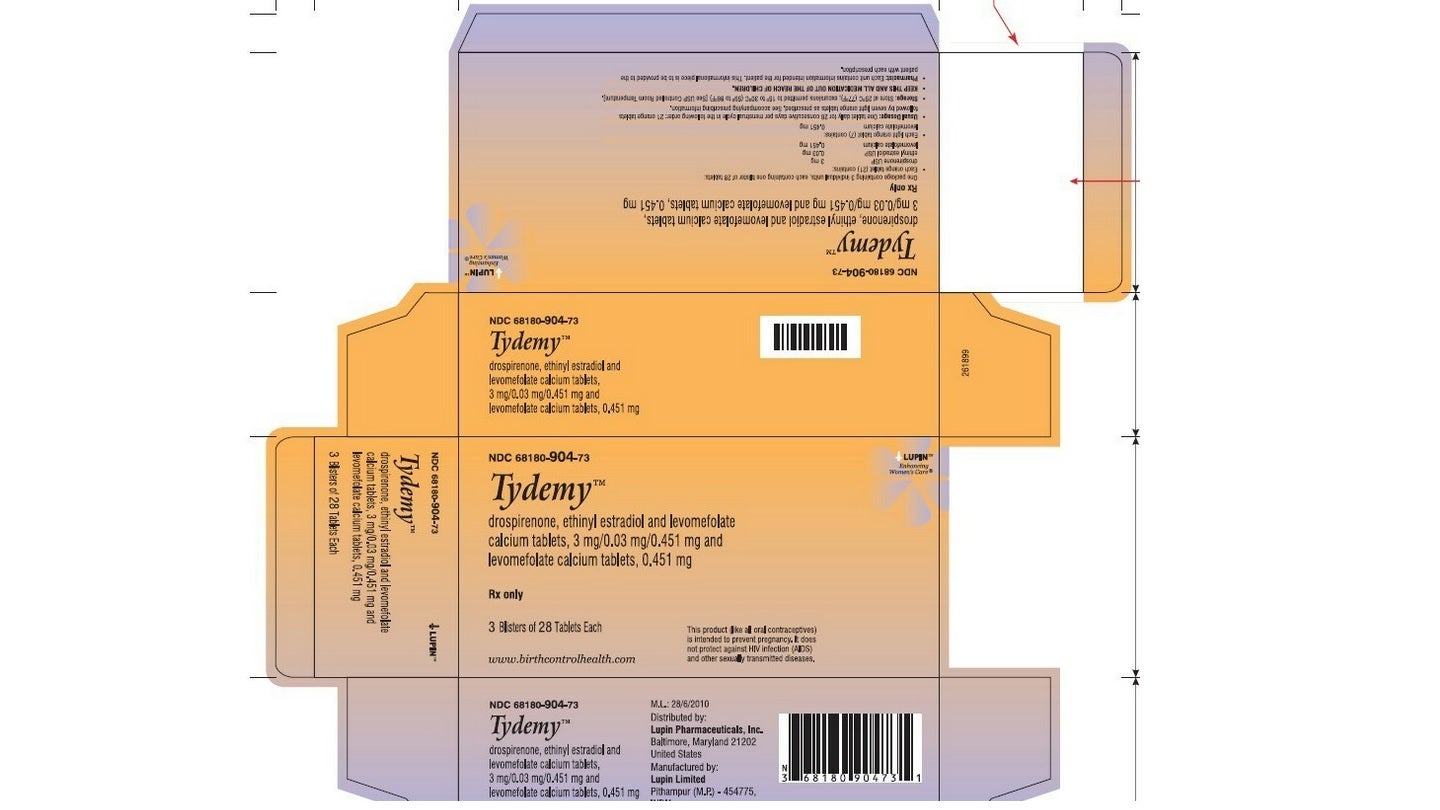
Lupin Pharmaceuticals has announced the voluntary recall of two lots of the pregnancy prevention drug Tydemy (drospirenone, ethinyl estradiol and levomefolate calcium tablets 3mg/0.03mg/0.451mg and levomefolate calcium tablets 0.451mg) in the US.
Tydemy is an estrogen/progestin oral contraceptive (COC) intended for women who wish to prevent pregnancy and for increasing folate levels in those who choose an oral contraceptive.
The tablets have been recalled because of out-of-specification (OOS) test results at the 12-month stability time point.
One lot in particular, L200183, tested low for ascorbic acid (an inactive ingredient), and also high for a known impurity.
Lupin is recalling the two lots due to a significant reduction in their inactive content (ascorbic acid), which may potentially impact the product’s effectiveness and result in unexpected pregnancies.
Tydemy is delivered in blister packs containing 28 tablets each. A single blister was packed into a pouch, along with a printed sleeve, a pack insert labelled with the day and an oxygen absorber sachet (Stabilox).
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataThe three pouches were packed in a single carton. The lots were then supplied across the US to wholesalers, mail-order pharmacies, drug chains and supermarkets.
Adverse reactions or quality problems with this product can be reported to the US Food and Drug Administration (FDA) MedWatch Adverse Event Reporting Programme online by regular mail or by fax.
So far, Lupin has not received any reports of adverse events associated with the recalled lots.