Precigen Receives the US FDA’s IND Clearance of PRGN-2009 + Pembrolizumab for the Treatment of Recurrent or Metastatic Cervical Cancer
PharmaShots
JUNE 1, 2023
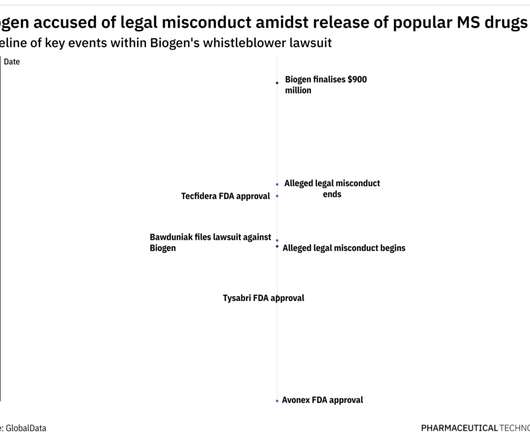
Shots: The US FDA has cleared the IND application to initiate a P-II study evaluating PRGN-2009 (off-the-shelf AdenoVerse immunotherapy) + pembrolizumab vs pembrolizumab monotx. while the secondary objectives incl. while the secondary objectives incl.












































Let's personalize your content